Question: How much heat (in kJ ) is evolved in converting 2.00mol of steam at 132C to ice at 41C ? The heat capacity of steam
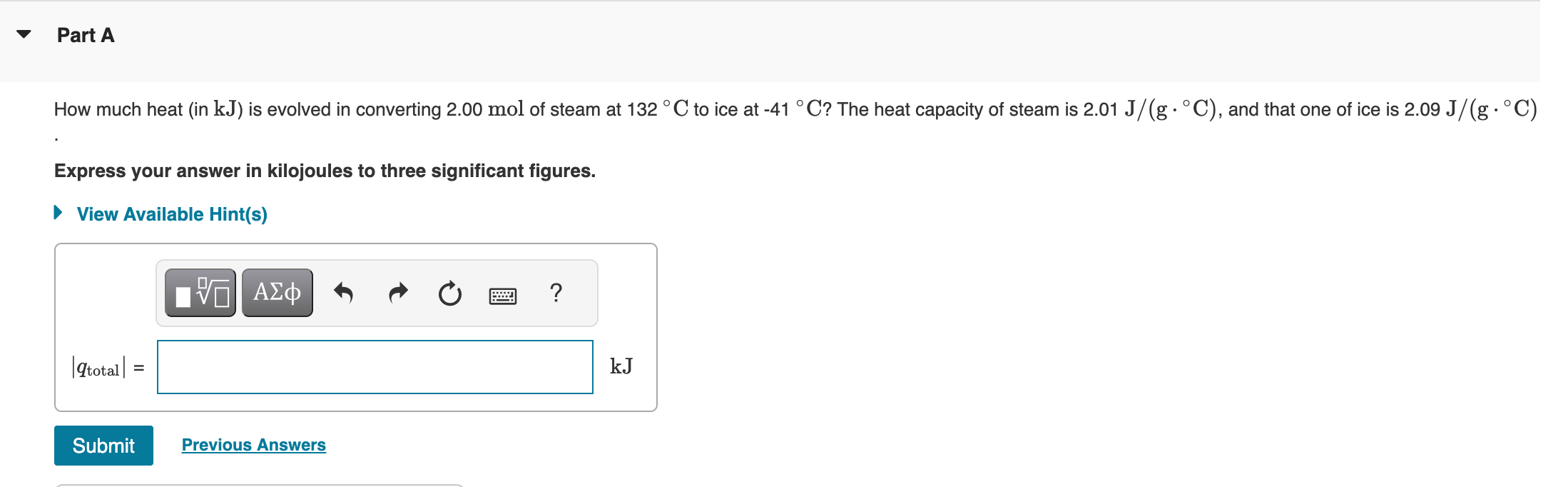
How much heat (in kJ ) is evolved in converting 2.00mol of steam at 132C to ice at 41C ? The heat capacity of steam is 2.01J/(gC ), and that one of ice is 2.09J/(gC ) Express your answer in kilojoules to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


