Question: How much heat is needed to convert 9 3 6 . g of ice at - 1 0 . 0 C to steam at 1
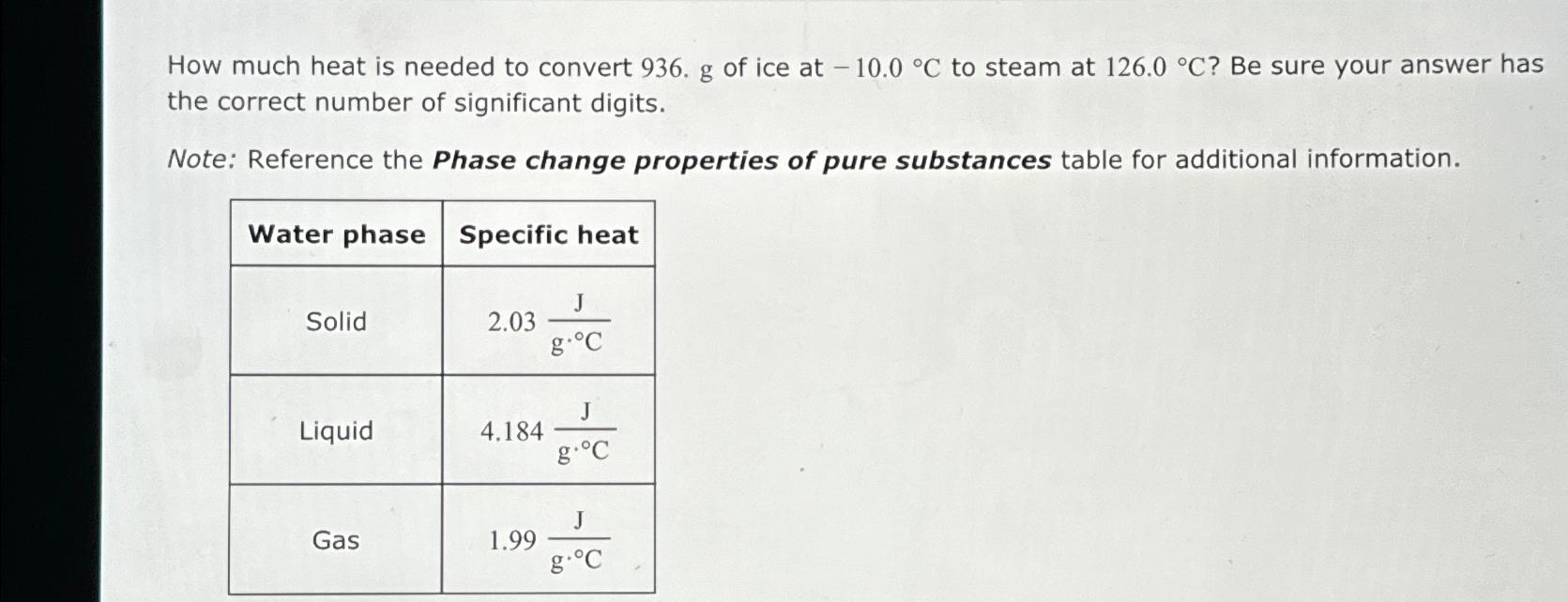
How much heat is needed to convert of ice at to steam at Be sure your answer has the correct number of significant digits.
Note: Reference the Phase change properties of pure substances table for additional information.
tableWater phase,Specific heatSolid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


