Question: How much time is required for the concentration to decrease? A certain reaction has the following general form: aAbB At a particular temperature and [A]0=3.50102M,
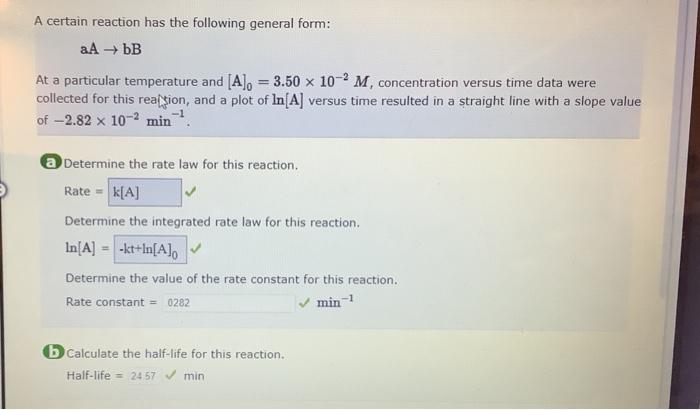
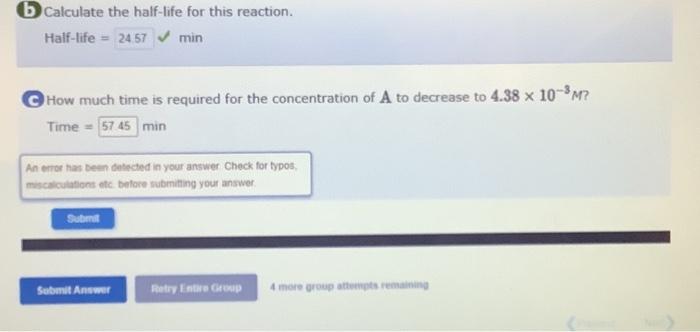
A certain reaction has the following general form: aAbB At a particular temperature and [A]0=3.50102M, concentration versus time data were collected for this rea/sion, and a plot of ln[A] versus time resulted in a straight line with a slope value of 2.82102min1. a Determine the rate law for this reaction. Rate = Determine the integrated rate law for this reaction. ln[A]= Determine the value of the rate constant for this reaction. Rate constant = min1 (b) Calculate the half-life for this reaction. Half-life =2457min b) Calculate the half-life for this reaction. Half-life= How much time is required for the concentration of A to decrease to 4.38103M ? Time=min An error has been detected in your answer Check for typos, miscatculations otc. before submining your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


