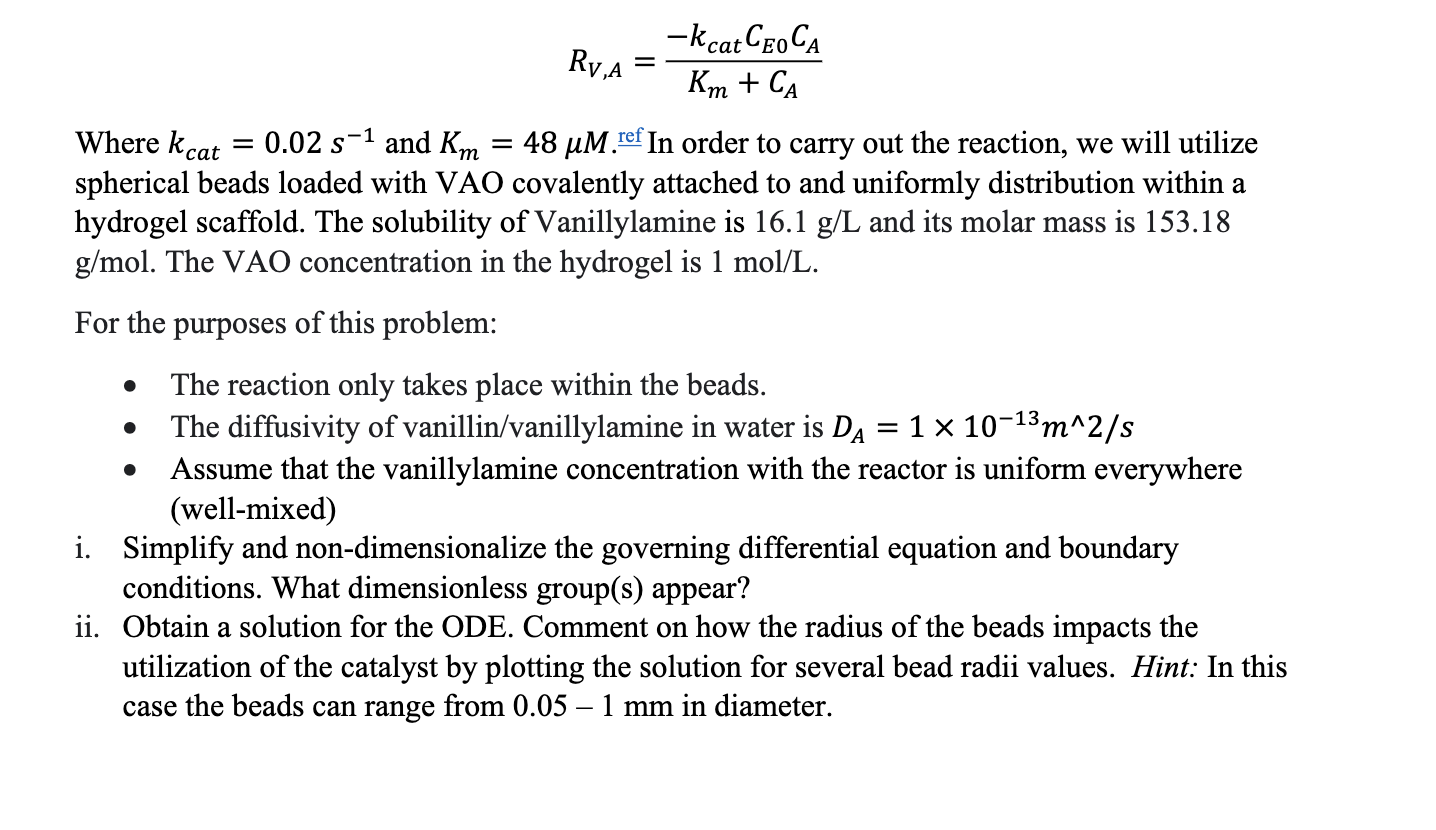
Question: how to non - dimensionalize and solve this question? R V , A = - k c a t C E 0 C A K
how to nondimensionalize and solve this question?
Where and ref In order to carry out the reaction, we will utilize
spherical beads loaded with VAO covalently attached to and uniformly distribution within a
hydrogel scaffold. The solubility of Vanillylamine is and its molar mass is gmol The VAO concentration in the hydrogel is molL
For the purposes of this problem:
The reaction only takes place within the beads.
The diffusivity of vanillinvanillylamine in water is
Assume that the vanillylamine concentration with the reactor is uniform everywhere
wellmixed
i Simplify and nondimensionalize the governing differential equation and boundary
conditions. What dimensionless groups appear?
ii Obtain a solution for the ODE. Comment on how the radius of the beads impacts the
utilization of the catalyst by plotting the solution for several bead radii values. Hint: In this
case the beads can range from in diameter.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


