Question: How to solve 1 4 . 2 8 . Consider the reactions 1 2 N 2 ( g ) + 1 2 O 2 (
How to solve
Consider the reactions
If these reactions come to equilibrium after combustion in an internalcombustion engine at and bar, estimate the mole fractions of and present for mole fractions of nitrogen and oxygen in the combustion products of and
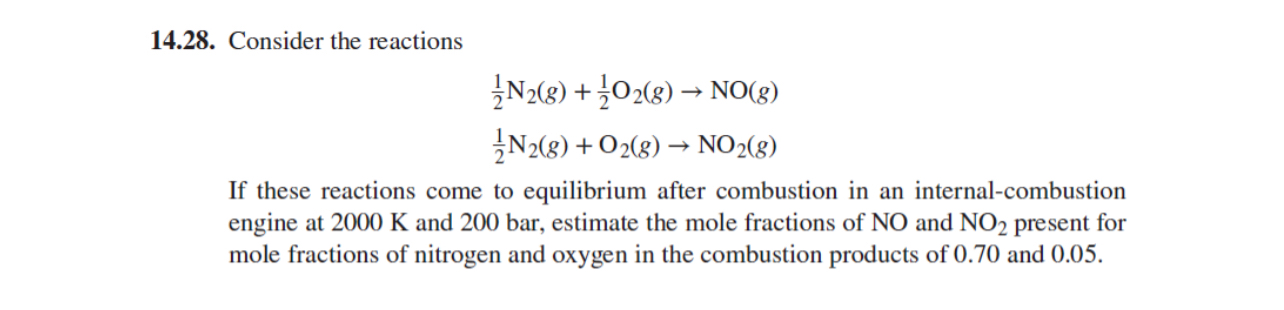
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


