Question: How to solve 1 9 . 2 6 * For an ideal gas C V and C p are different because of the work W
How to solve
For an ideal gas and are different because of the work associated with the volume change for a constantpressure process. To explore the difference between and for a liquid or a solid, consider the process in which mol of ethanol is warmed from to while the applied pressure remains a constant atm The molar mass of ethanol is a What is the mass of mol of ethanol? b How much heat enters the ethanol? c How much work is done by the ethanol because of its thermal expansion? d What is the ratio
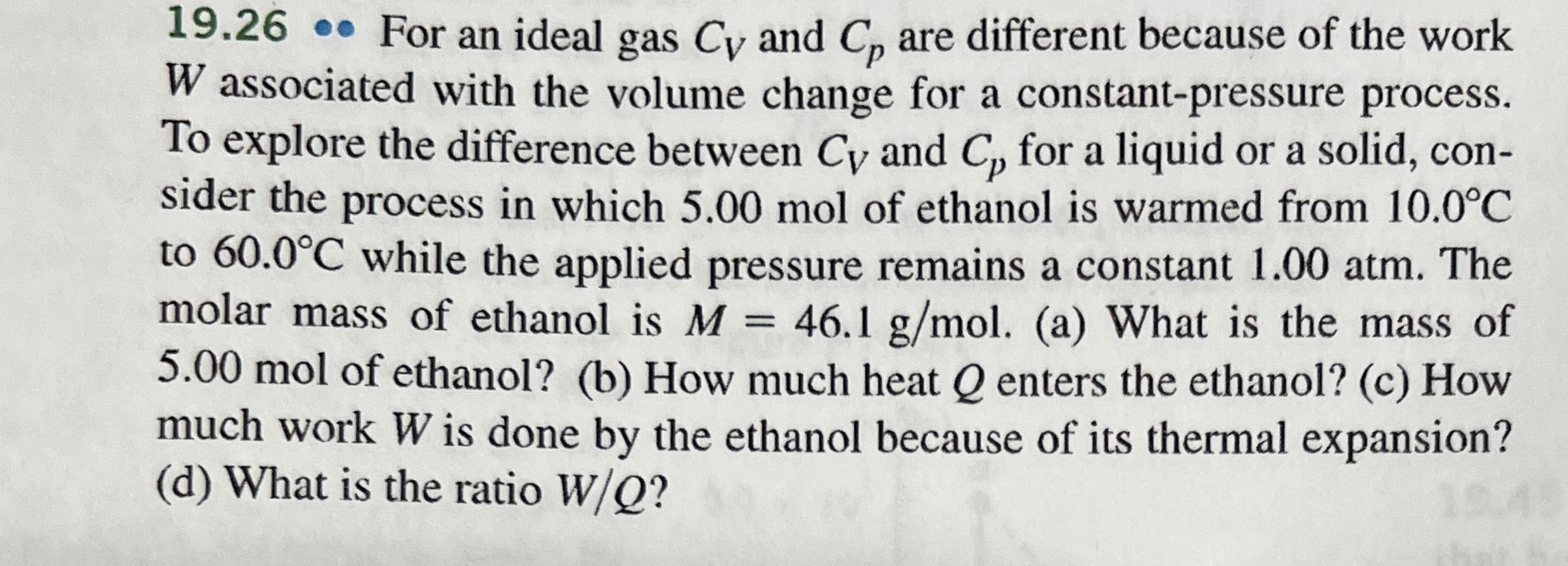
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


