Question: How to solve C and D? Here we calculate various points on the velocity distrubtion curve for helium gas at T=1000K. We assume the distribution
How to solve C and D?
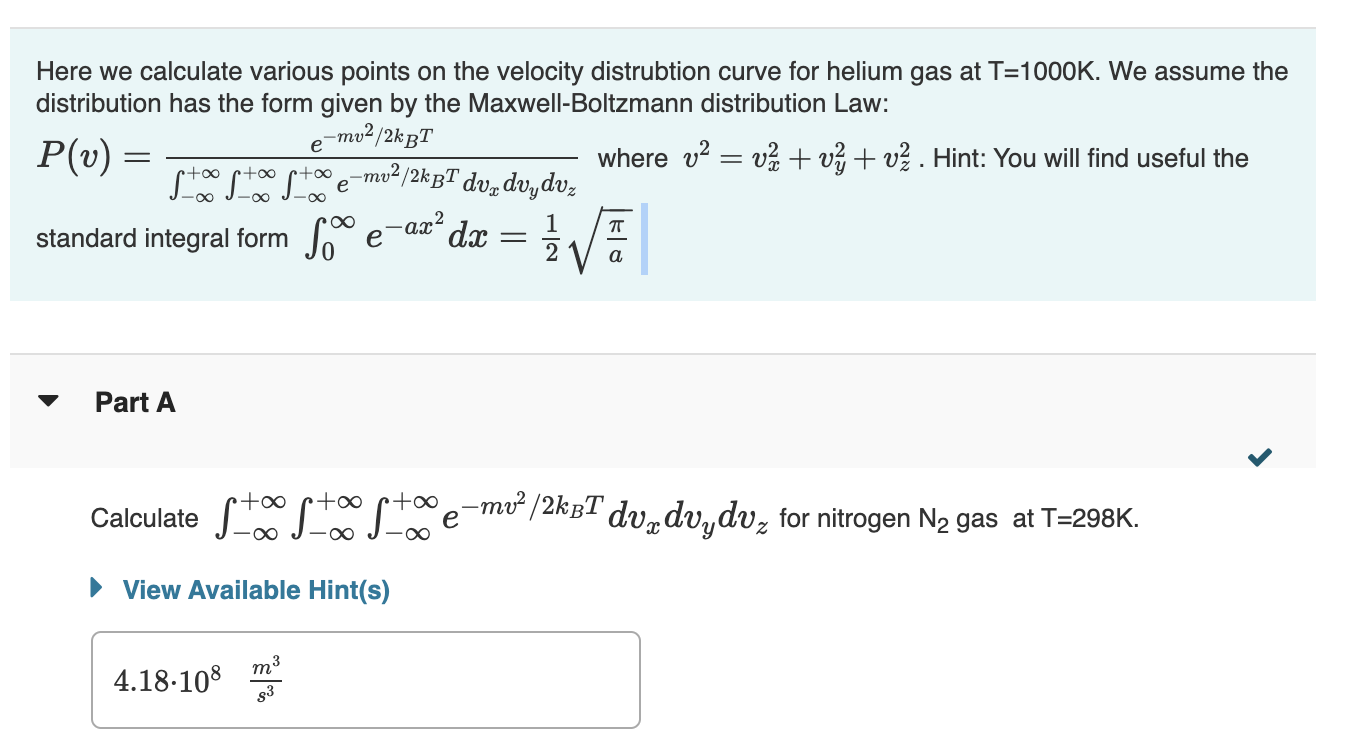
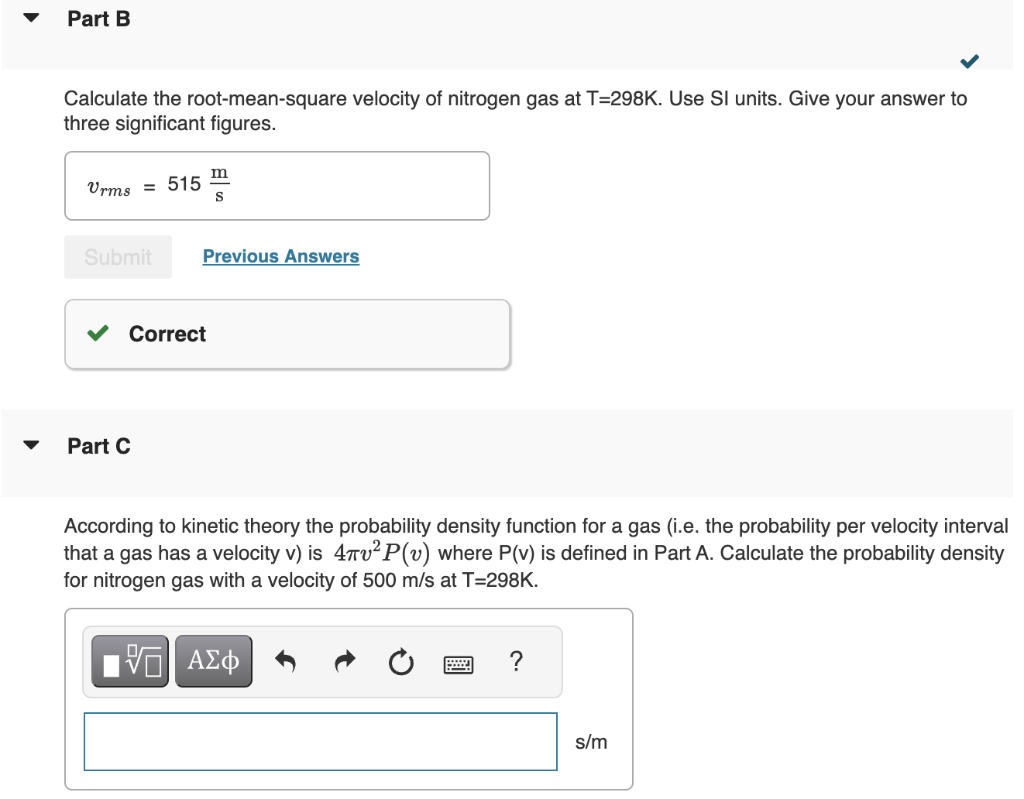

Here we calculate various points on the velocity distrubtion curve for helium gas at T=1000K. We assume the distribution has the form given by the Maxwell-Boltzmann distribution Law: P(v)=+++emv2/2kBTdvxdvydvzemv2/2kBT where v2=vx2+vy2+vz2. Hint: You will find useful the standard integral form 0eax2dx=21a Part A Calculate +++emv2/2kBTdvxdvydvz for nitrogen N2 gas at T=298K. View Available Hint(s) Calculate the root-mean-square velocity of nitrogen gas at T=298K. Use SI units. Give your answer to three significant figures. Part C According to kinetic theory the probability density function for a gas (i.e. the probability per velocity interval that a gas has a velocity v ) is 4v2P(v) where P(v) is defined in Part A. Calculate the probability density for nitrogen gas with a velocity of 500m/s at T=298K. Calculate the ratio of the number of nitrogen moleculse at T=298K having a velocity of 500m/s versus 750 m/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


