Question: how to solve the problem? During a reversible process, 396 kJ of heat is added to 1.264 kg of a certain gas while the volume
how to solve the problem?
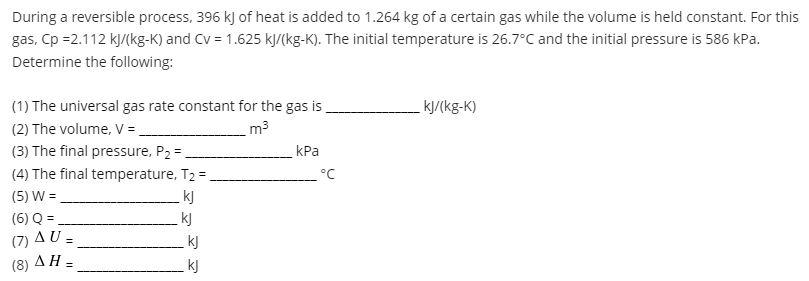
During a reversible process, 396 kJ of heat is added to 1.264 kg of a certain gas while the volume is held constant. For this gas, Cp =2.112 kJ/(kg-K) and CV = 1.625 kJ/(kg-K). The initial temperature is 26.7C and the initial pressure is 586 kPa. Determine the following: kJ/(kg-K) m3 (1) The universal gas rate constant for the gas is (2) The volume, V = (3) The final pressure, P2 = kPa (4) The final temperature, T2 = C (5) W = k] (6) Q = kj (7) U = kj kj (8)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


