Question: (a) The Lewis structure for methane, CH4, is given to the right. Using the JSmol tool, -identify which of the following would be a
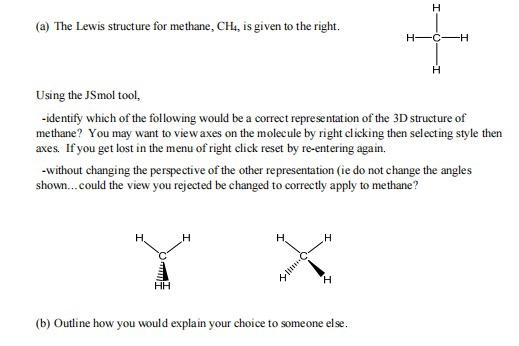
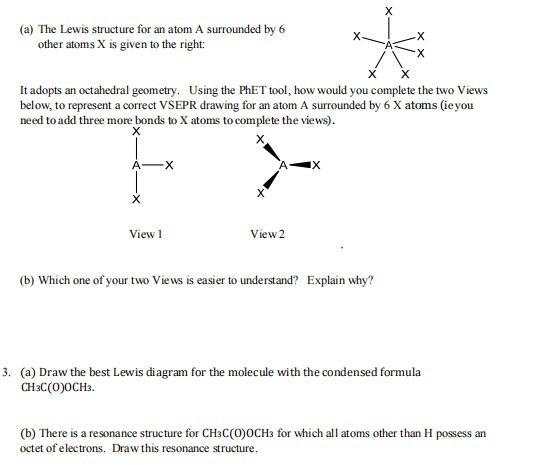
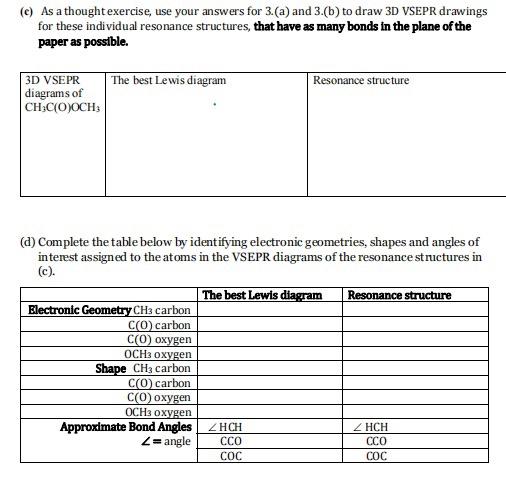
(a) The Lewis structure for methane, CH4, is given to the right. Using the JSmol tool, -identify which of the following would be a correct representation of the 3D structure of methane? You may want to view axes on the molecule by right clicking then selecting style then axes. If you get lost in the menu of right click reset by re-entering again. -without changing the perspective of the other representation (ie do not change the angles shown...could the view you rejected be changed to correctly apply to methane? H H X H H + C -H Q.. (b) Outline how you would explain your choice to someone else. X -X * It adopts an octahedral geometry. Using the PhET tool, how would you complete the two Views below, to represent a correct VSEPR drawing for an atom A surrounded by 6 X atoms (ie you need to add three more bonds to X atoms to complete the views). (a) The Lewis structure for an atom A surrounded by 6 other atoms X is given to the right: X View 1 X View 2 IX (b) Which one of your two Views is easier to understand? Explain why? 3. (a) Draw the best Lewis diagram for the molecule with the condensed formula CH3C(O)OCH3. (b) There is a resonance structure for CH3C(O)OCH3 for which all atoms other than H possess an octet of electrons. Draw this resonance structure. (e) As a thought exercise, use your answers for 3. (a) and 3.(b) to draw 3D VSEPR drawings for these individual resonance structures, that have as many bonds in the plane of the paper as possible. 3D VSEPR diagrams of CHC(O)OCH The best Lewis diagram (d) Complete the table below by identifying electronic geometries, shapes and angles of interest assigned to the atoms in the VSEPR diagrams of the resonance structures in (c). Electronic Geometry CH3 carbon C(O) carbon C(O) oxygen OCH3 oxygen Shape CH3 carbon C(O) carbon Resonance structure The best Lewis diagram C(O) oxygen OCH3 oxygen Approximate Bond Angles ZHCH
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


