Question: how to solve these problems 8. Buffer A 0.24 M Glycine Jan22 30 % viv Methanol mL. dH20 up to 0.75 L final volume (you
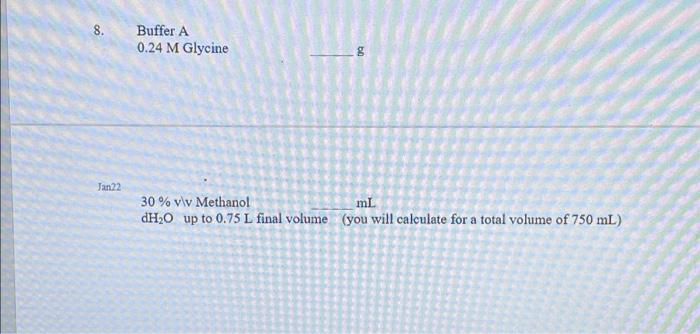

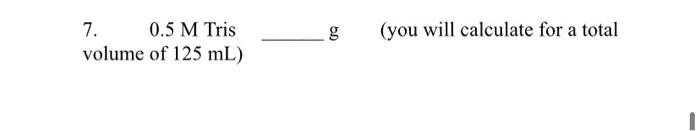
8. Buffer A 0.24 M Glycine Jan22 30 % viv Methanol mL. dH20 up to 0.75 L final volume (you will calculate for a total volume of 750 mL) Useful Information -- Molecular Mass (Formula Weights): Tris 121.12 g/mol Glycine 75.07 g/mol 7. 0.5 M Tris volume of 125 mL) bun (you will calculate for a total
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


