Question: how to solve this problem? 2. Below is given the FTIR data of 1:1 (by weight) mixture of ethylene carbonate and dimethyl carbonate. Absorbance P
how to solve this problem?
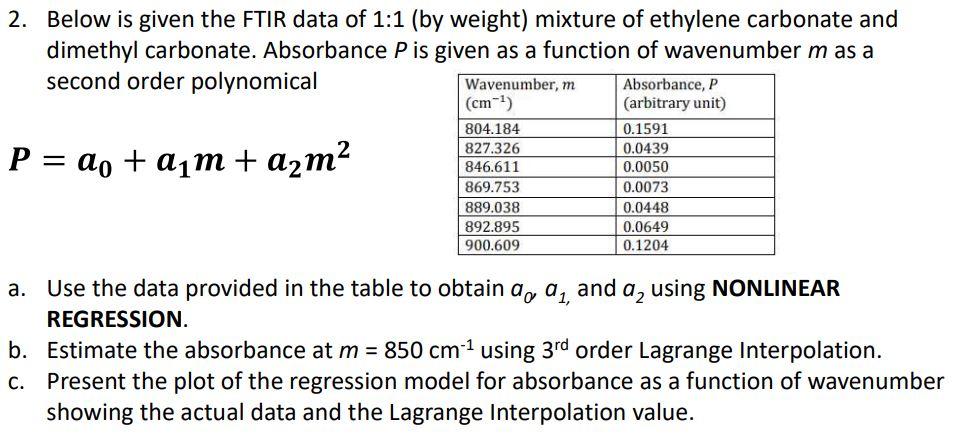
2. Below is given the FTIR data of 1:1 (by weight) mixture of ethylene carbonate and dimethyl carbonate. Absorbance P is given as a function of wavenumber mas a second order polynomical Wavenumber, m Absorbance, P (arbitrary unit) 804.184 0.1591 827.326 0.0439 P = ao + aim + a2m2 (cm) 846.611 869.753 889.038 892.895 900.609 0.0050 0.0073 0.0448 0.0649 0.1204 a. Use the data provided in the table to obtain a, a,, and a, using NONLINEAR REGRESSION b. Estimate the absorbance at m = 850 cm-1 using 3rd order Lagrange Interpolation. C. Present the plot of the regression model for absorbance as a function of wavenumber showing the actual data and the Lagrange Interpolation value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


