Question: how to solve this using an ice table? Consider the following reaction: H2S(g)H2(g)+S(g)Kp=1.67107 PH2Sinitial=3.00atm and PH2initial=0.00450atm. Determine PH2Seq,PH2cq, and PSc
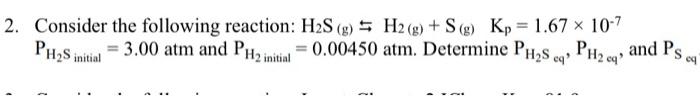
Consider the following reaction: H2S(g)H2(g)+S(g)Kp=1.67107 PH2Sinitial=3.00atm and PH2initial=0.00450atm. Determine PH2Seq,PH2cq, and PSc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


