Question: How was this calculated Example 21.11 The force on a water molecule The water molecule H2O has a permanent dipole moment of magnitude 6.2 x
How was this calculated
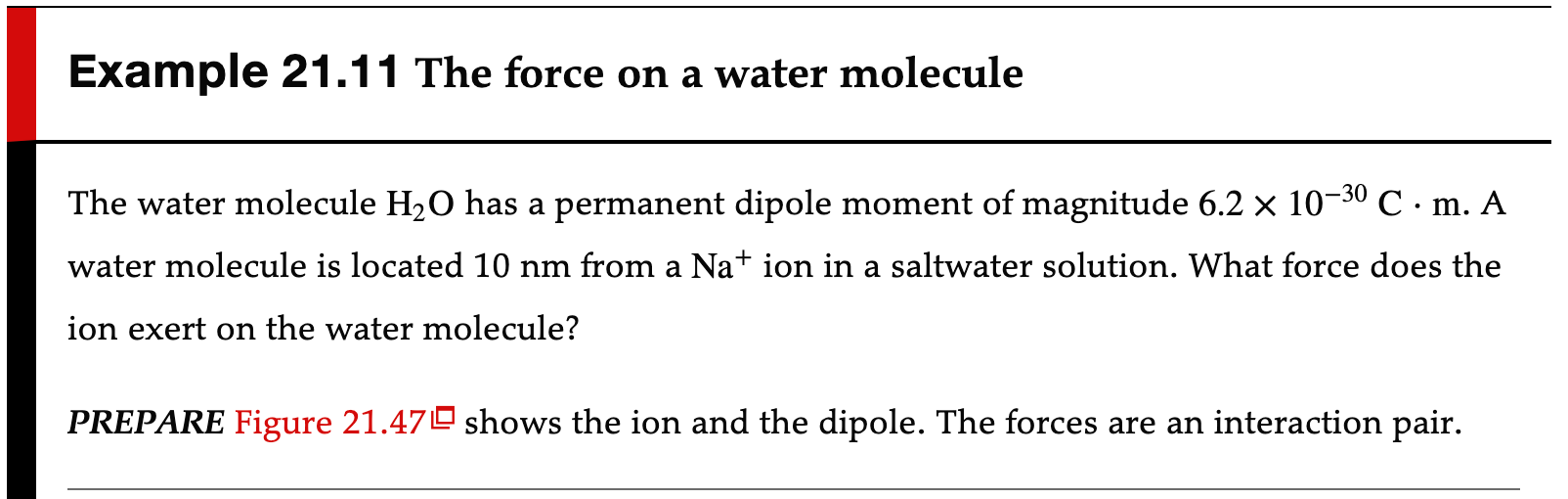
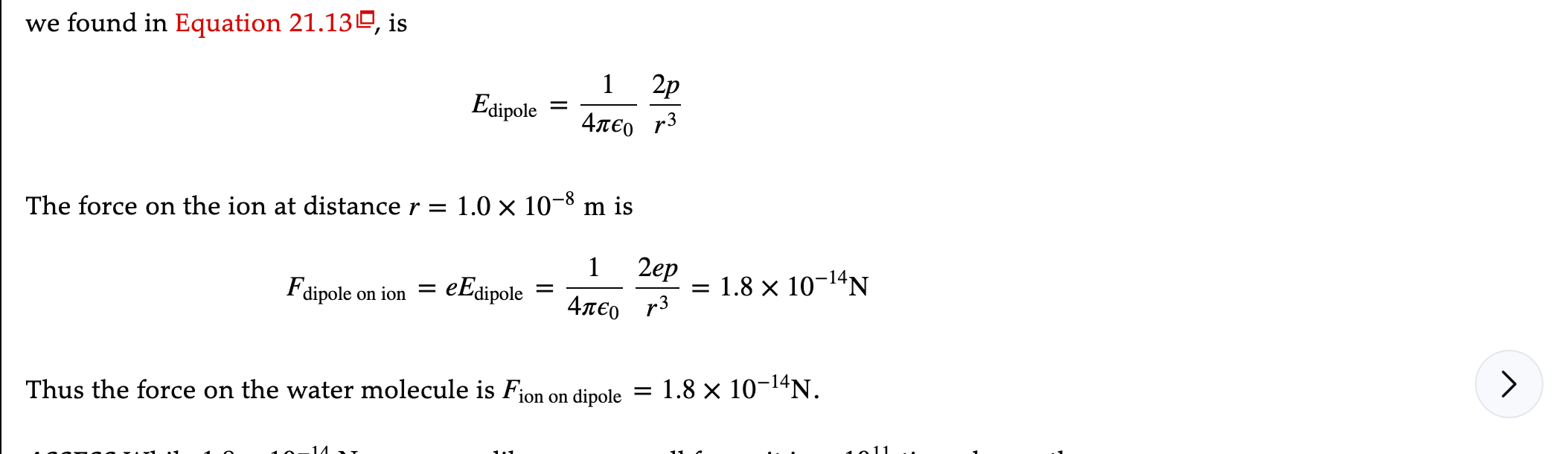
Example 21.11 The force on a water molecule The water molecule H2O has a permanent dipole moment of magnitude 6.2 x 10-30 C . m. A water molecule is located 10 nm from a Nation in a saltwater solution. What force does the ion exert on the water molecule? PREPARE Figure 21.47 shows the ion and the dipole. The forces are an interaction pair.we found in Equation 21.139, is 2p Edipole =7 4TEO The force on the ion at distance r = 1.0 x 10-8 m is 1 2ep = 1.8 x 10-14N Fdipole on ion = eEdipole = ATEO > Thus the force on the water molecule is Fion on dipole = 1.8 x 10-14N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


