Question: HW Ch 2, part 2 i 1 Problem 02.040 - Stirred heated water in closed pan Saved 14.28 points Water is being heated in
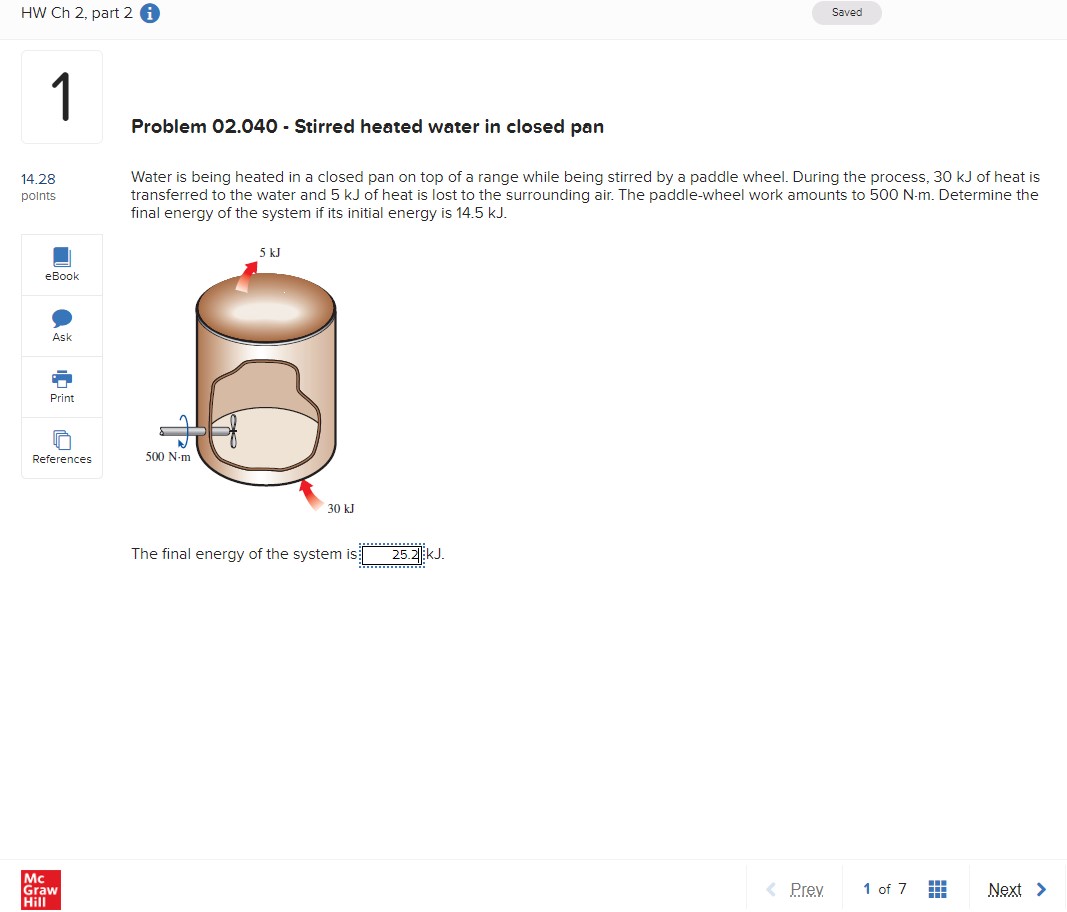
HW Ch 2, part 2 i 1 Problem 02.040 - Stirred heated water in closed pan Saved 14.28 points Water is being heated in a closed pan on top of a range while being stirred by a paddle wheel. During the process, 30 kJ of heat is transferred to the water and 5 kJ of heat is lost to the surrounding air. The paddle-wheel work amounts to 500 N-m. Determine the final energy of the system if its initial energy is 14.5 kJ. eBook Ask Print References 500 N-m Mc Graw Hill 5 kJ 30 kJ The final energy of the system is 25.2 kJ. < Prev 1 of 7 Next >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


