Question: HW2. Hi please help with each question in photo. Will give thumbs up! How much thermal energy (in ]) is required to boil 2.30 kg
HW2. Hi please help with each question in photo. Will give thumbs up!
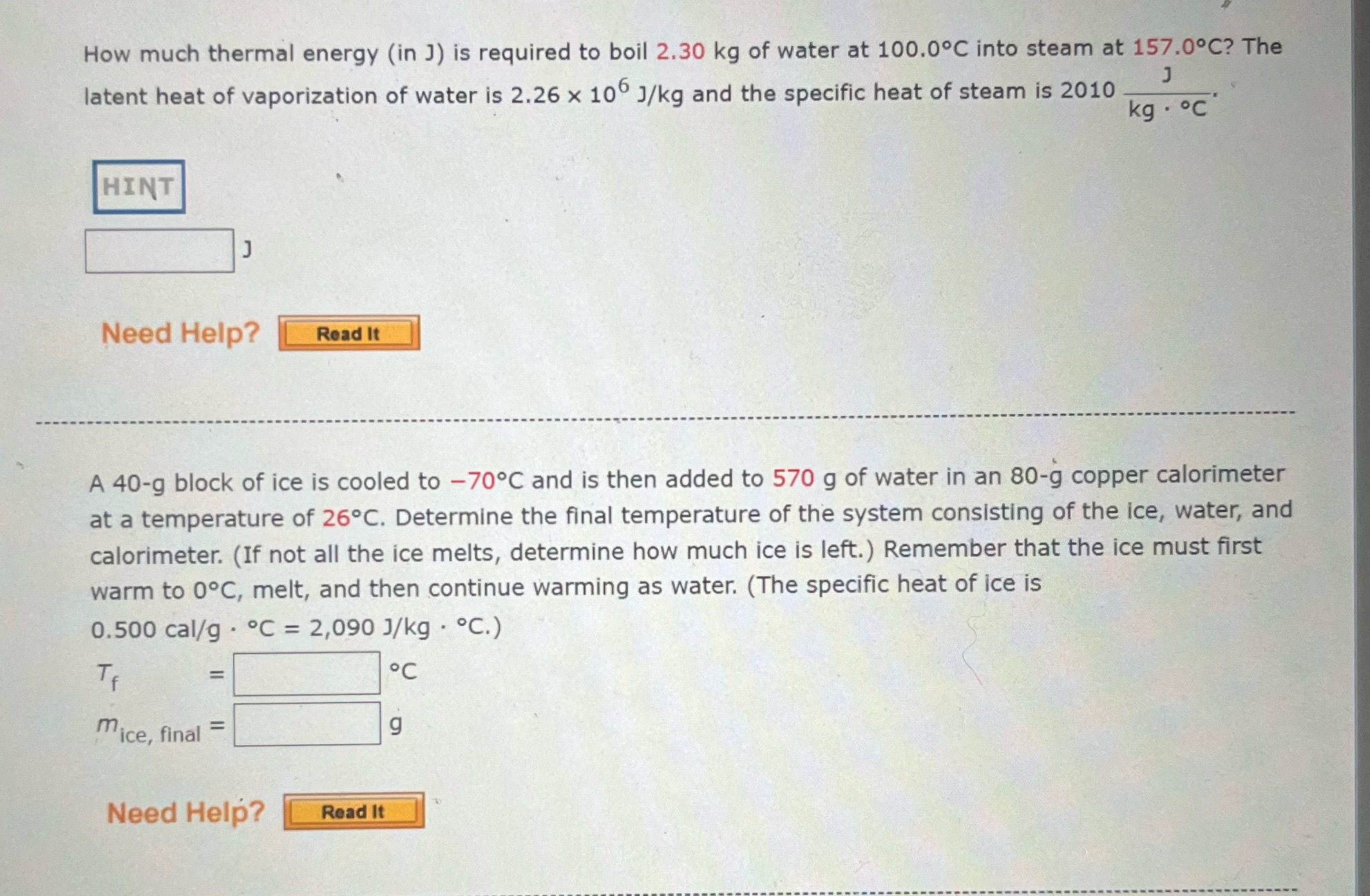
How much thermal energy (in ]) is required to boil 2.30 kg of water at 100.0C into steam at 157.0.C? The latent heat of vaporization of water is 2.26 x 10 J/kg and the specific heat of steam is 2010 J kg . c HINT Need Help? Read It A 40-g block of ice is cooled to -70C and is then added to 570 g of water in an 80-g copper calorimeter at a temperature of 26C. Determine the final temperature of the system consisting of the ice, water, and calorimeter. (If not all the ice melts, determine how much ice is left. ) Remember that the ice must first warm to OC, melt, and then continue warming as water. (The specific heat of ice is 0.500 cal/g . C = 2,090 J/kg . C.) T OC mice, final g Need Help? Read It
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


