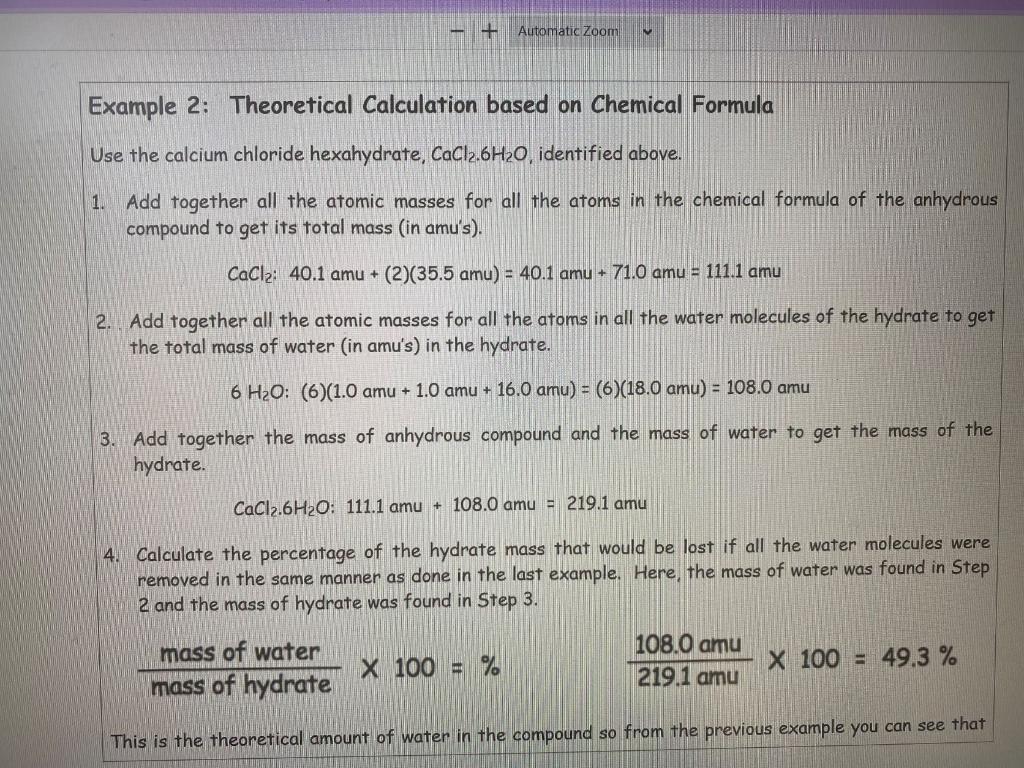
Question: Hydrates Lab Table- 2022 Automatic Zoom Example 2: Theoretical Calculation based on Chemical Formula Use the calcium chloride hexahydrate, CaCl2.6H20, identified above. 1. Add together
Hydrates Lab Table- 2022
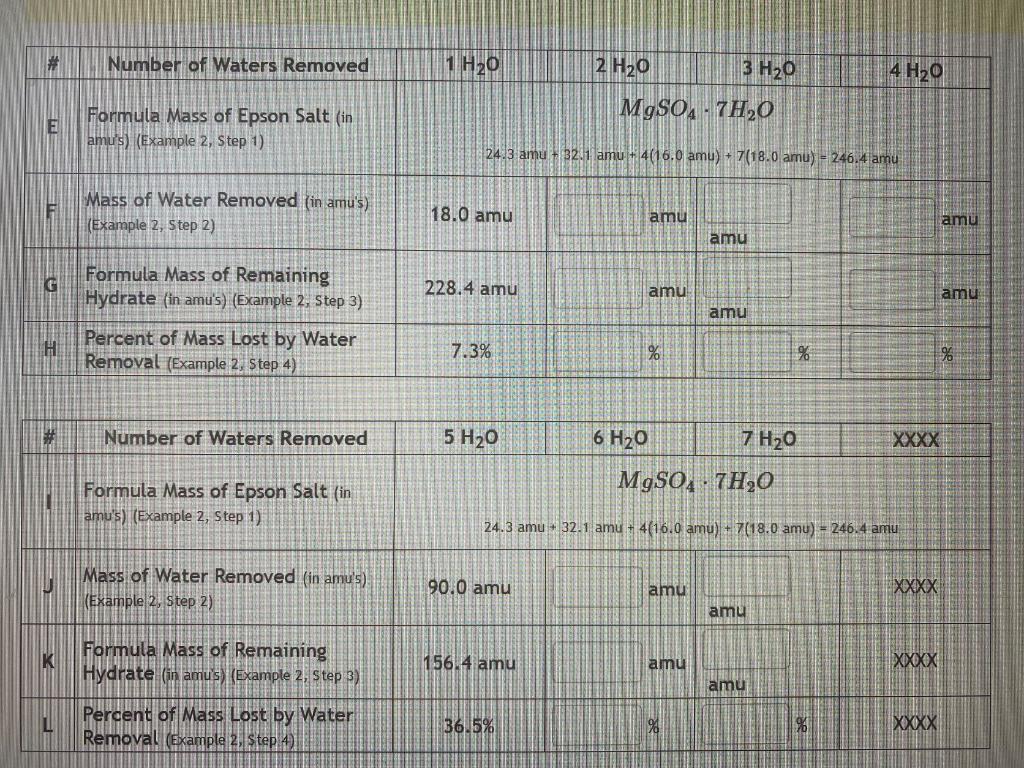
Automatic Zoom Example 2: Theoretical Calculation based on Chemical Formula Use the calcium chloride hexahydrate, CaCl2.6H20, identified above. 1. Add together all the atomic masses for all the atoms in the chemical formula of the anhydrous compound to get its total mass (in amu's). CaCl2: 40.1 amu + (2)(35.5 amu) = 40.1 amu + 71.0 amu = 111.1 amu 2. Add together all the atomic masses for all the atoms in all the water molecules of the hydrate to get the total mass of water (in amu's) in the hydrate. 6 H2O: (6)(1.0 amu + 1.0 amu + 16.0 amu) = (6)(18.0 amu) = 108.0 amu 3. Add together the mass of anhydrous compound and the mass of water to get the mass of the hydrate. CaCl2.6H2O: 111.1 amu + 108.0 amu = 219.1 amu 4. Calculate the percentage of the hydrate mass that would be lost if all the water molecules were removed in the same manner as done in the last example. Here, the mass of water was found in Step 2 and the mass of hydrate was found in Step 3. mass of water mass of hydrate X 100 = % 108.0 amu 219.1 amu X 100 = 49.3 % This is the theoretical amount of water in the compound so from the previous example you can see that # Number of Waters Removed 1 H20 2 H30 3 H20 4 H20 MgSO4 - 7H20 E Formula Mass of Epson Salt (in amu's) (Example 2, Step 1) 24.3 amu + 32.1 Tamu + 4(16.0 amu) + 7 (18.0 amu) = 246.4 amu FI Mass of Water Removed (in amu's) (Example 2, Step 2) 18.0 amu amu amu amu G 228.4 amu amu amu Formula Mass of Remaining Hydrate (in amu's) (Example 2. Step 3) Percent of Mass Lost by Water Removal (Example 2. Step 4) amu H 7.3% % % 96 Number of Waters Removed 5 H20 6 H20 7 H20 MgSO4 - 7H30 Formula Mass of Epson Salt (in amu's) (Example 2, Step 1) 24.3 amu - 32.1 amu + 4(16.0 amu) + 7(18.0 amu) = 246.4 amu U Mass of Water Removed (in amu's) (Example 2, Step 2) 90.0 amu amu XXXX amu K Formula Mass of Remaining Hydrate (in amus) (Example 2, Step 3) 156.4 amu amu XXXX amu Percent of Mass Lost by Water Removal (Example 2, Step42 36.5% % XXXX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


