Question: Hydrogen atoms emit light when electrons move from higher to lower energy levels. Imagine the first three energy levels of hydrogen atoms as shown. Select
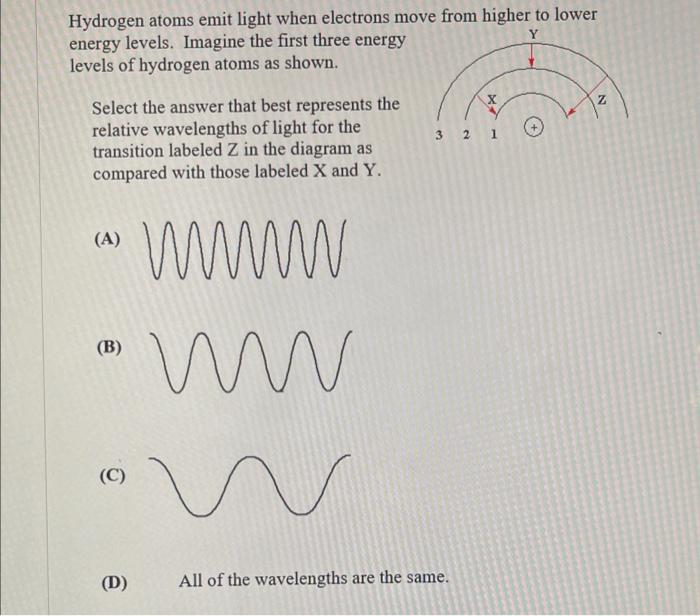
Hydrogen atoms emit light when electrons move from higher to lower energy levels. Imagine the first three energy levels of hydrogen atoms as shown. Select the answer that best represents the relative wavelengths of light for the transition labeled Z in the diagram as compared with those labeled X and Y. (A) (B) (C) (D) All of the wavelengths are the same
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


