Question: (i) A metal having a cubic structure has a density of 2.6g/cm3, an atomic weight of 87.62g/mol, and a lattice parameter of 6.0849A. Determine the
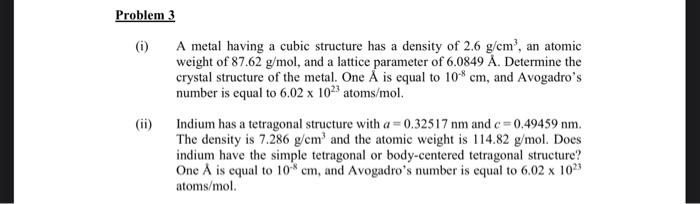
(i) A metal having a cubic structure has a density of 2.6g/cm3, an atomic weight of 87.62g/mol, and a lattice parameter of 6.0849A. Determine the crystal structure of the metal. One A is equal to 108cm, and Avogadro's number is equal to 6.021023 atoms /mol. (ii) Indium has a tetragonal structure with a=0.32517nm and c=0.49459nm. The density is 7.286g/cm3 and the atomic weight is 114.82g/mol. Does indium have the simple tetragonal or body-centered tetragonal structure? One A is equal to 108cm, and Avogadro's number is equal to 6.021023 atoms/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


