Question: I am really Lost please help me understand this from the begining. 7. The reaction 2NO(g)+2H2(g)N2(g)+2H2O(g) was studied at 904C, and the data in the
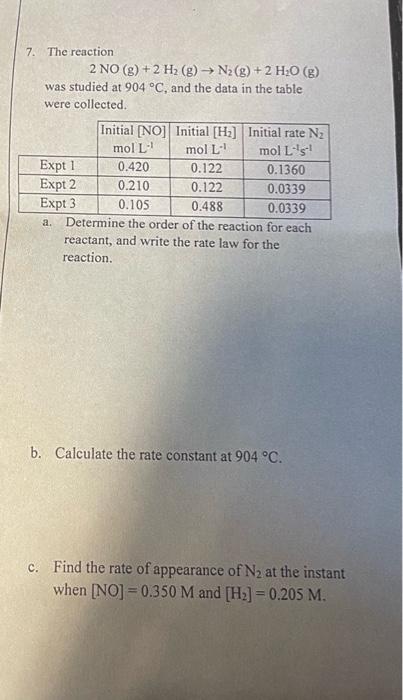
7. The reaction 2NO(g)+2H2(g)N2(g)+2H2O(g) was studied at 904C, and the data in the table were collected. a. Determme the order of the reaction for each reactant, and write the rate law for the reaction. b. Calculate the rate constant at 904C. c. Find the rate of appearance of N2 at the instant when [NO]=0.350M and [H2]=0.205M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


