Question: I am stuck on this problem A model for H2O is shown in the chem3D window. H2O has bent geometry. This geometry is sometimes called
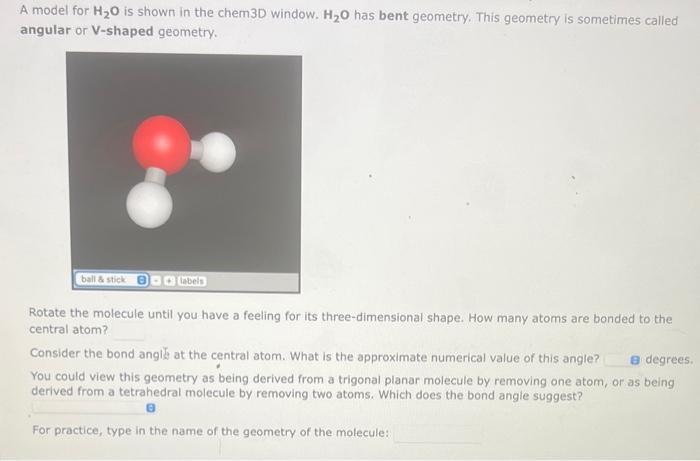
A model for H2O is shown in the chem3D window. H2O has bent geometry. This geometry is sometimes called angular or V-shaped geometry. Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom? Consider the bond angl at the central atom. What is the approximate numerical value of this angle? You could view this geometry as being derived from a trigonal planar molecule by removing one atom, or as being derived from a tetrahedral molecule by removing two atoms. Which does the bond angle suggest? For practice, type in the name of the geometry of the molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


