Question: i) Assess whether the flash process is possible under these conditions. (ii) Assuming ideal gas behaviour, calculate the product compositions in vapour and liquid outlet
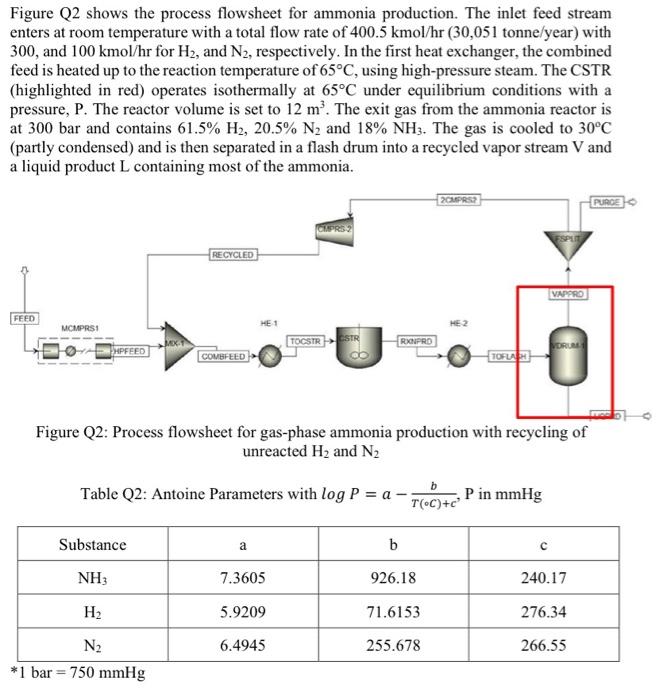
Figure Q2 shows the process flowsheet for ammonia production. The inlet feed stream enters at room temperature with a total flow rate of 400.5 kmol/hr (30,051 tonne/year) with 300, and 100 kmol/hr for H2, and N2, respectively. In the first heat exchanger, the combined feed is heated up to the reaction temperature of 65C, using high-pressure steam. The CSTR (highlighted in red) operates isothermally at 65C under equilibrium conditions with a pressure, P. The reactor volume is set to 12 m. The exit gas from the ammonia reactor is at 300 bar and contains 61.5% H2, 20.5% N2 and 18% NH3. The gas is cooled to 30C (partly condensed) and is then separated in a flash drum into a recycled vapor stream V and a liquid product L containing most of the ammonia. 2CMPRS PURCE RECYCLED VAPPRE FEED ME 1 MCMPRSI HE 2 TOCSTR CSTR RXPRD MPFEED COMBFEED TOFLAR Figure Q2: Process flowsheet for gas-phase ammonia production with recycling of unreacted H2 and N2 b Table Q2: Antoine Parameters with log P = a P in mmHg T(C)+c Substance a b NH 7.3605 926.18 240.17 H2 5.9209 71.6153 276.34 6.4945 255.678 266.55 N2 *1 bar = 750 mmHg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


