Question: I ATTACHED THE PROCEDURE FOR PART II B . THANK YOU TO THE KIND HEARTED TUTOR Activity No. 13 WORKSHEET METAL IONS IN SOLUTION Part


I ATTACHED THE PROCEDURE FOR PART II B . THANK YOU TO THE KIND HEARTED TUTOR
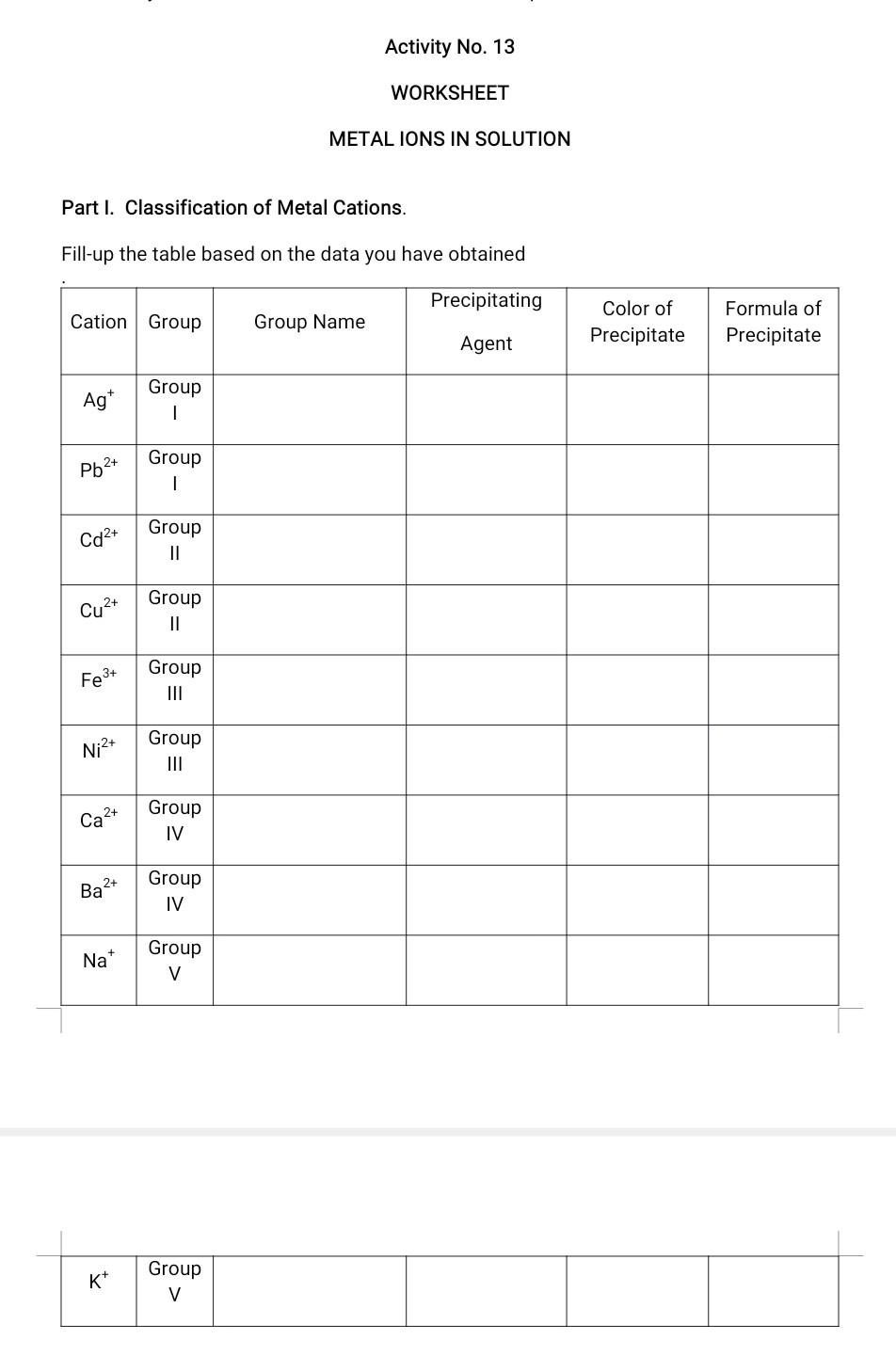
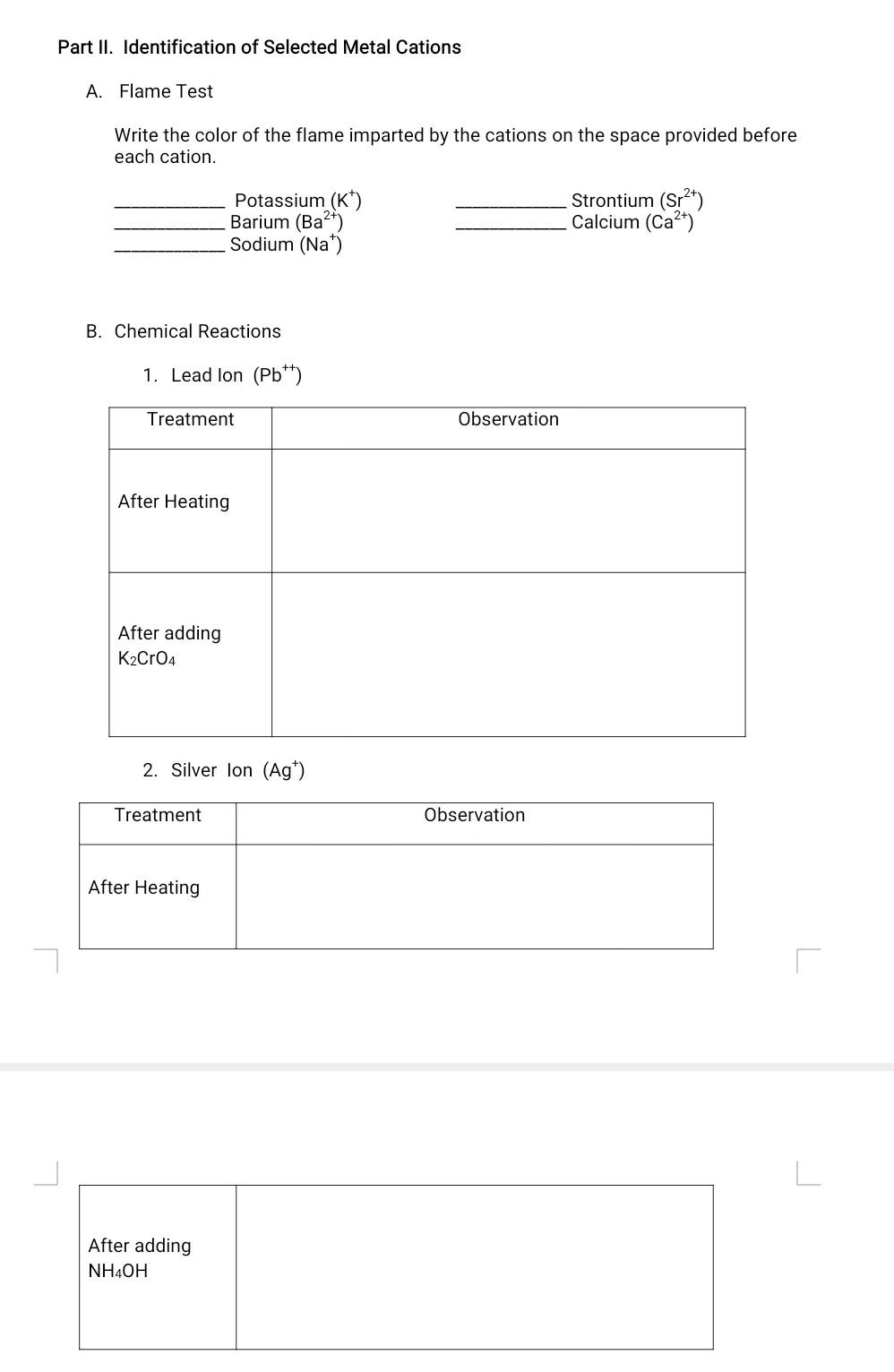
Activity No. 13 WORKSHEET METAL IONS IN SOLUTION Part I. Classification of Metal Cations. Fill-up the table based on the data you have obtained Precipitating Cation Group Group Name Color of Precipitate Formula of Precipitate Agent Ag Group . Group Pb2+ Cd2+ Group 11 2+ Cu- Group II Group Fe3+ Group Ni2+ III 2+ Ca Group IV 2+ Ba Group IV Na Group V Kt Group V Part II. Identification of Selected Metal Cations A. Flame Test Write the color of the flame imparted by the cations on the space provided before each cation. Potassium (K) Barium (Ba2+) Sodium (Na+) Strontium (Sr2+) Calcium (Ca2+) B. Chemical Reactions 1. Lead lon (Pb++) Treatment Observation After Heating After adding K2Cr04 2. Silver Ion (Ag) Treatment Observation After Heating After adding NH4OH GUIDE QUESTIONS 1. Give the group number where the cation belongs. a. Mercurous ion (Hg22+) gives a white precipitate with 0.1 M HCI. b. Bismuth ion (Bit) does not give a precipitate with HCl but gives a black precipitate with thioacetamide in the presence of HCI. c. Ammonium ion (NH4+) does not give precipitate with any precipitating agent. d. Magnesium ion (Mg2+) does not give a precipitate with HCl or thioacetamide but give a white solid product with sodium carbonate. e. Cobalt ion (Co2t) does not give a solid product with HCl and thioacetamide unless the solution is treated with excess ammonium hydroxide. 2. Which can be used as a precipitating agent for group I cation in the absence of hydrochloric acid? HCIO or NaCl? Explain. L 3. When testing for acidity with litmus paper why should you not put the litmus paper into the solution? 4. Why is it possible to use potassium sulfide instead of thioacetamide in precipitating the group II and group III cations? 5. In the test for ammonium ion why should you take care that the litmus paper is not wet with the solution in the evaporating dish? 6. Give two ways on how will you distinguish silver chloride from lead (II) chloride. CONCLUSION Part II. Identification of Selected Cations. A. Flame Tests 1. Place 2 mL of denatured alcohol in an evaporating dish. 2. Using a lighted match stick light the denatured alcohol in the evaporating dish then quickly drop a saturated solution of sodium chloride to the flame. 3. Observe and describe the color imparted by the flame. 4. After thorough observation, wash the evaporating dish and dry well. L 5. Repeat the procedure using saturated solutions of KCI, SrCl2, BaCl2 and CaCl2 instead of sodium chloride. B. Chemical Reactions 1) Test for Lead Cation (Dissolution of Precipitate) a. Heat the test tube containing the precipitate of lead ion in Part I. b. Observe what happens to the precipitate. c. Add 2 to 3 drops of 1.0 M K2CrO4 while the test tube is hot. d. Observe what happens. 2) Test for Silver Cations (Complex Formation) a. Heat the test tube containing silver ion. Is the result the same as the lead ion? b. Let the test tube cool down then add 6.0 M NH4OH dropwise until the precipitate dissolves. 3) Test for Ammonium lon (Evolution of Ammonia gas). a.Place 10 drops of 1.0 M NH4OH in an evaporating dish and add 1.0 mL of 6.0 M sodium hydroxide. b. Place a piece of moistened piece of litmus paper. Be sure the litmus paper is not moistened by the solution in the evaporating dish. c. Cover the evaporating dish with the watch glass with litmus paper and gently heat the solution near boiling. d. Observe the change in color of the litmus paper as the fumes from the sample touches the litmus paper
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


