Question: i cant figure out how to do these 2 problems under calculation 1 and 2 the absorbance was 1- 0.045 2- 0.047 3- 0. 073
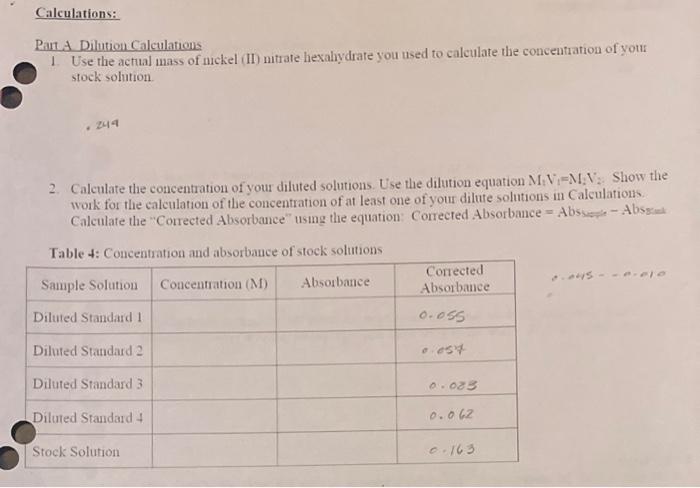
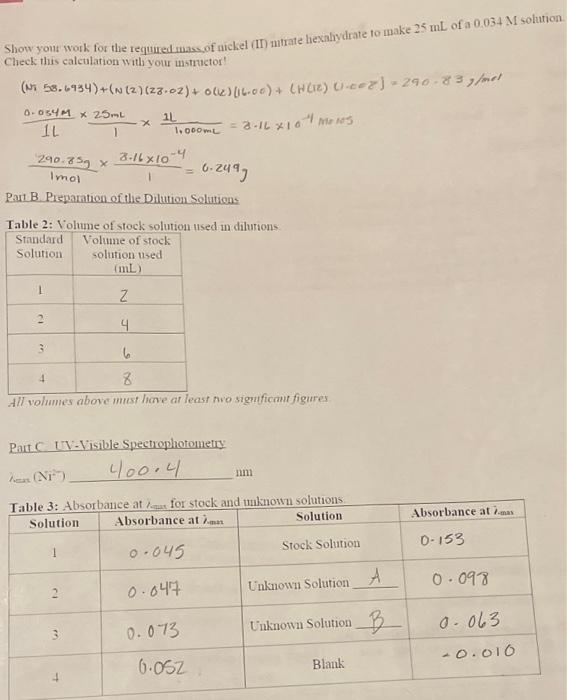
Pant Dilution Calculations 1 Use the actual mass of nckel (II) nitrate hexalydrate you used to calculate the concentration of your stock solution. .244 2. Calenlate the concentration of your diluted solutions. Use the dilution equation M1V1=M1N2 : Show the work for the calculation of the concentration of at least one of your dilute solutions in Calculations. Calculate the "Corrected Absorbance" using the equation: Corrected Absorbance = Abs we-e - Abssaik Table 4: Concentration and absorbance of stock solutions Show yous work for the required mass of mickel (II) mirate hexaliydrate to make 25mL of a 0.034M solution Check this calculation with your mstructor! (N.58.6934)+(N(2)(28.02)+o(k)(16.00)+(w(12)(1.0=2)=290.83,fol1L0.034M125mL1.000mL1L=8.16101/mons1mol290.85g18.16104=0.249J Patt B Prepantion of the Dilution Solutions Table 2: Volume of stock solution used in dilutions All vohumes above must have at least no siguficmit figures Pait C V - Visible spectrophotometry Nan(Ni2+) 400,4 nm. Pant Dilution Calculations 1 Use the actual mass of nckel (II) nitrate hexalydrate you used to calculate the concentration of your stock solution. .244 2. Calenlate the concentration of your diluted solutions. Use the dilution equation M1V1=M1N2 : Show the work for the calculation of the concentration of at least one of your dilute solutions in Calculations. Calculate the "Corrected Absorbance" using the equation: Corrected Absorbance = Abs we-e - Abssaik Table 4: Concentration and absorbance of stock solutions Show yous work for the required mass of mickel (II) mirate hexaliydrate to make 25mL of a 0.034M solution Check this calculation with your mstructor! (N.58.6934)+(N(2)(28.02)+o(k)(16.00)+(w(12)(1.0=2)=290.83,fol1L0.034M125mL1.000mL1L=8.16101/mons1mol290.85g18.16104=0.249J Patt B Prepantion of the Dilution Solutions Table 2: Volume of stock solution used in dilutions All vohumes above must have at least no siguficmit figures Pait C V - Visible spectrophotometry Nan(Ni2+) 400,4 nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


