Question: I could use some help for this I didnt understand it thanks B) Suppose for the reaction SbCl3(g)+Cl2(g)=SbCl5(g) The following mechanism is correct: step1step2step3Cl2Cl.+SbCl3=SbCl4slowSbCl4+Cl2=SbCl5+Cl.=2Cl.fastFast[Cl]=k[Cl2]1/2 1-
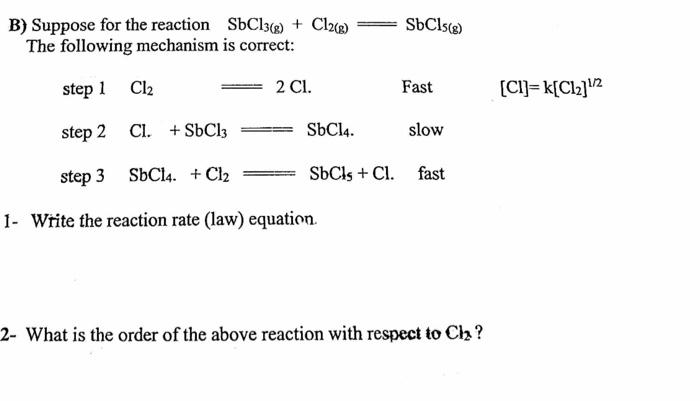
B) Suppose for the reaction SbCl3(g)+Cl2(g)=SbCl5(g) The following mechanism is correct: step1step2step3Cl2Cl.+SbCl3=SbCl4slowSbCl4+Cl2=SbCl5+Cl.=2Cl.fastFast[Cl]=k[Cl2]1/2 1- Write the reaction rate (law) equation. 2- What is the order of the above reaction with respect to Cl2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


