Question: I do not understand O.A. and R.A. in this case. Please provide an explanation while completing remaining of the table. Leave the, well portion of
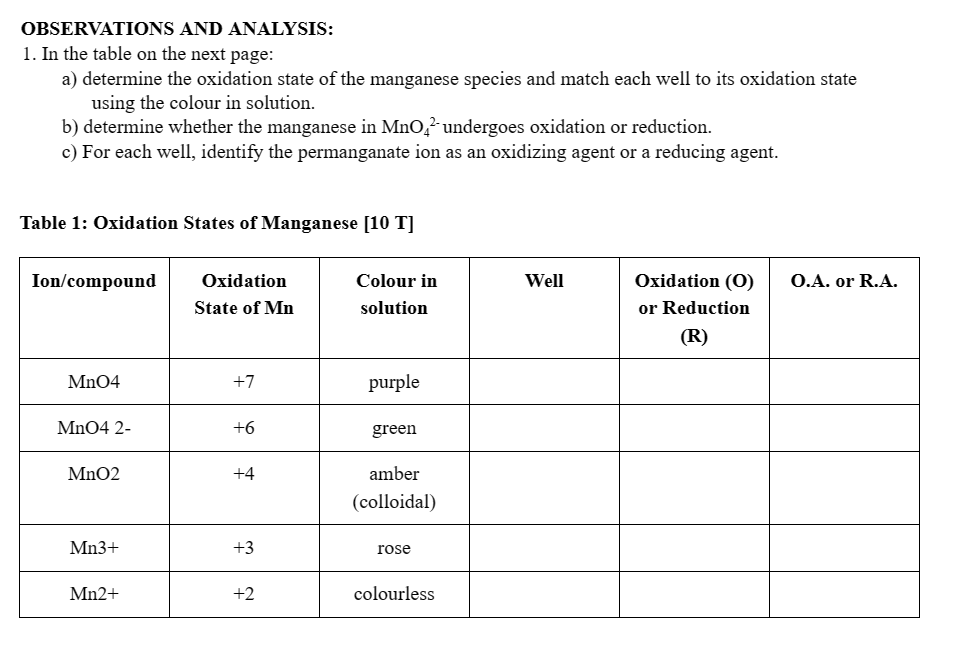
I do not understand O.A. and R.A. in this case. Please provide an explanation while completing remaining of the table. Leave the, "well" portion of the table empty.

Please explain.
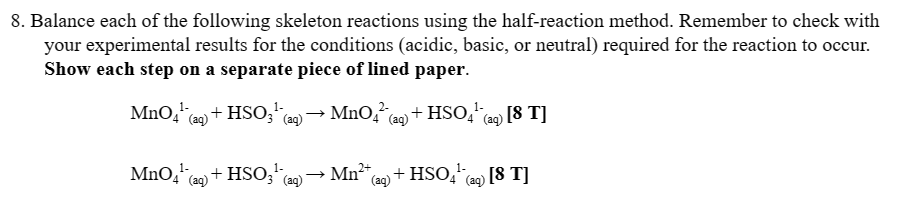
Please balance these two, I need to check my work to see if I am on the right track. Thank you
OBSERVATIONS AND ANALYSIS: 1. In the table on the next page: a) determine the oxidation state of the manganese species and match each well to its oxidation state using the colour in solution. b) determine whether the manganese in MnO42 undergoes oxidation or reduction. c) For each well, identify the permanganate ion as an oxidizing agent or a reducing agent. Table 1: Oxidation States of Manganese [10 T] 5. What is the relationship between the oxidation state of manganese produced and the pH? [1T] 8. Balance each of the following skeleton reactions using the half-reaction method. Remember to check with your experimental results for the conditions (acidic, basic, or neutral) required for the reaction to occur. Show each step on a separate piece of lined paper
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


