Question: I dont understand how can we solve and draw the orbitals from the character table? Consider the angular OX2 molecule. Each of the terminal X
I dont understand how can we solve and draw the orbitals from the character table?
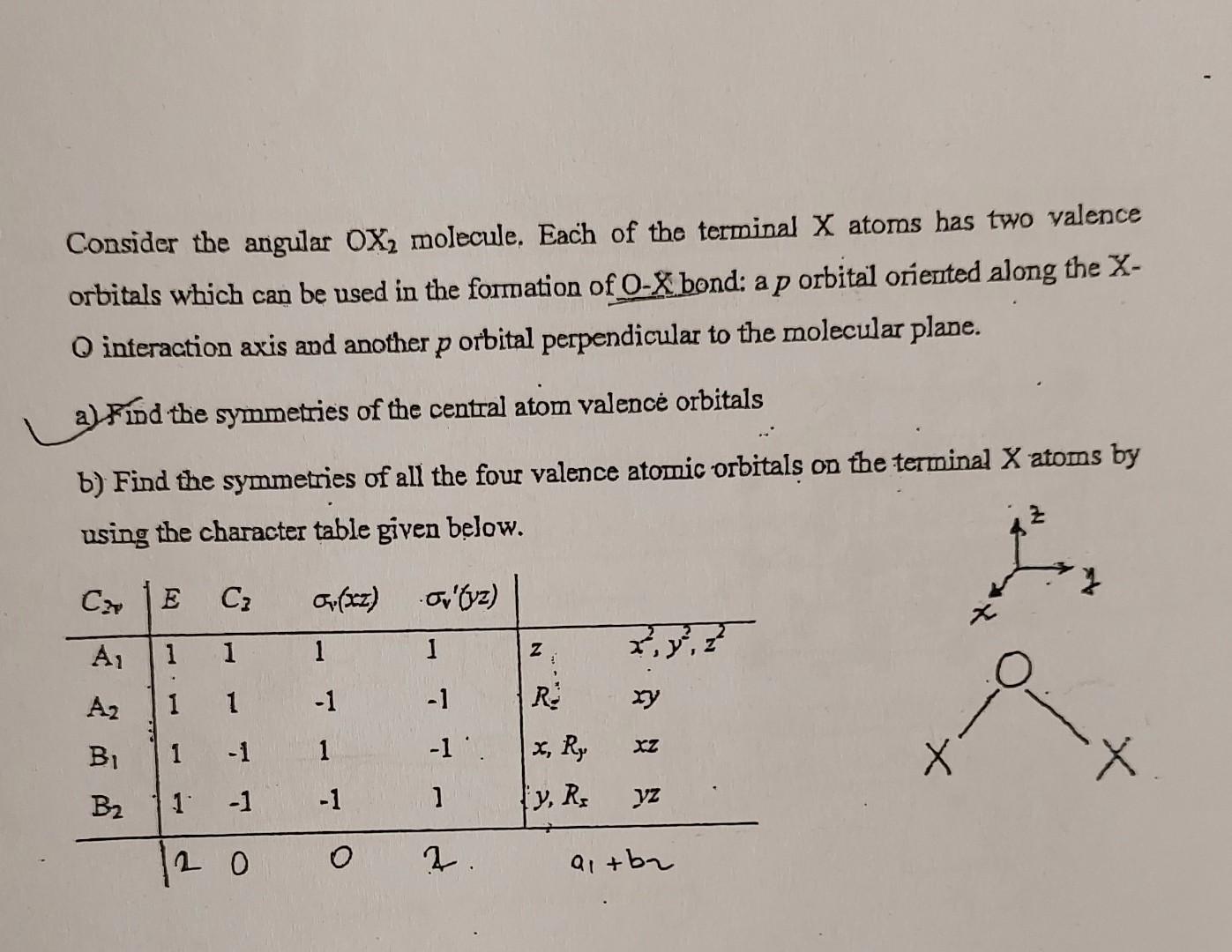
Consider the angular OX2 molecule. Each of the terminal X atoms has two valence orbitals which can be used in the fomation of O - X bond: a p orbital oriented along the X O interaction axis and another p orbital perpendicular to the molecular plane. a) Find the symmetries of the central atom valence orbitals b) Find the symmetries of all the four valence atomic orbitals on the terminal X atoms by using the character table given below
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


