Question: I filled out as much as i can, pls solve the column for Psyr(Pa) and the two that follow it. USING THE DATA IN RED!!
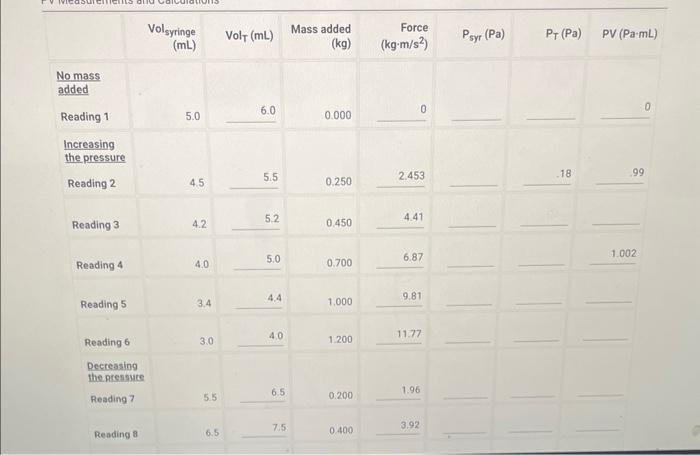
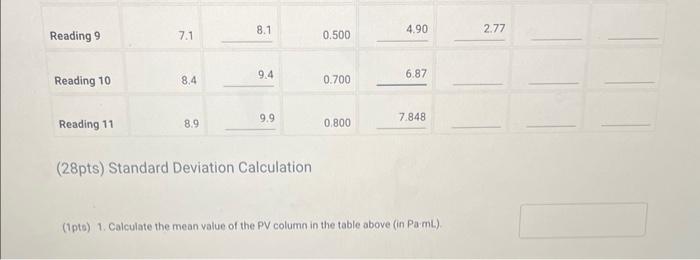
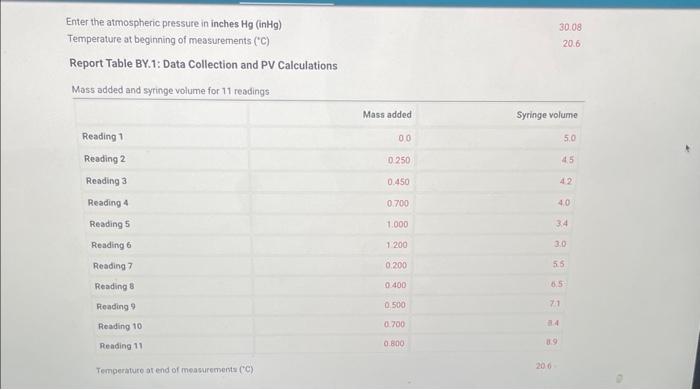
No mass added Reading 1 5.0 0.000 Increasing the pressure Reading 2 \begin{tabular}{llll} 4.5 & 5.5 & 0.250 & 2.453 \\ \hline \end{tabular} Reading 3 \begin{tabular}{llll} 4.2 & 5.2 & 0.450 & 4.41 \\ \hline \end{tabular} Reading 4 4.0 5.0 0.7006.87 \begin{tabular}{llll} 3.4 & 4.4 & 1.000 & 9.81 \\ \hline \end{tabular} Reading 6 3.0 4.0 1.20011.77 Decreasing the pressute Reading 7 \begin{tabular}{lll} 5.5 & 6.5 & 0.2001.96 \\ \hline 6.5 & 7.5 & 0.4003.92 \\ \hline \end{tabular} (28pts) Standard Deviation Calculation (1pto) 1. Calculate the mean value of the PV column in the table above (in Pa mL ). Enter the atmospheric pressure in inches Hg(inHg) Temperature at beginning of measurements ('C) Report Table BY.1: Data Collection and PV Calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


