Question: i got a solution for this problem, but i couldn't understand so i reload it 3 For the cell PtH2(1atm)HCl(0.0256molkg1AgClAg, the emf at 298K is
i got a solution for this problem, but i couldn't understand so i reload it
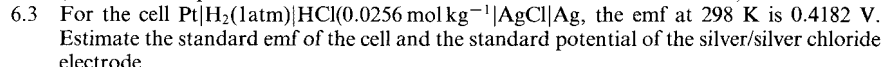
3 For the cell PtH2(1atm)HCl(0.0256molkg1AgClAg, the emf at 298K is 0.4182V. Estimate the standard emf of the cell and the standard potential of the silver/silver chloride electrode 3 For the cell PtH2(1atm)HCl(0.0256molkg1AgClAg, the emf at 298K is 0.4182V. Estimate the standard emf of the cell and the standard potential of the silver/silver chloride electrode
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


