Question: i got the last one right but the first two wrong Red gold is a gold-copper alloy used to make jewelry. A piece of jewelry
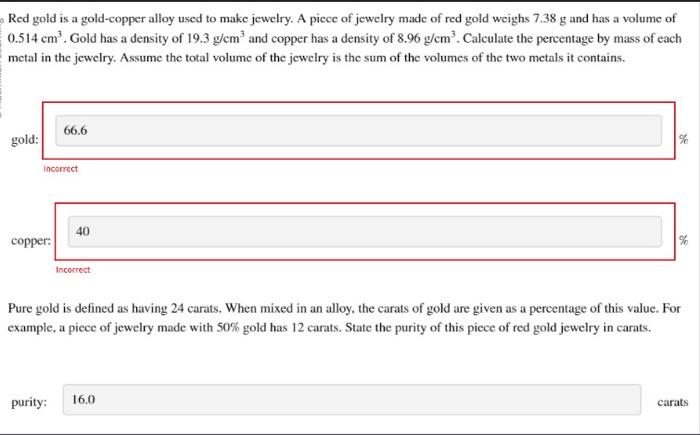
Red gold is a gold-copper alloy used to make jewelry. A piece of jewelry made of red gold weighs 7.38g and has a volume of 0.514cm3. Gold has a density of 19.3g/cm3 and copper has a density of 8.96g/cm3. Calculate the percentage by mass of each metal in the jewelry. Assume the total volume of the jewelry is the sum of the volumes of the two metals it contains. golk incorrect coppe incorrect Pure gold is defined as having 24 carats. When mixed in an alloy, the carats of gold are given as a percentage of this value. For example, a piece of jewelry made with 50% gold has 12 carats. State the purity of this piece of red gold jewelry in carats
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


