Question: I have a through d but need helping finding the rest a) 151.36 b) 75.68 c) 24.32 d) 50.66 A reaction and separator, are configured
 I have a through d but need helping finding the rest
I have a through d but need helping finding the rest
a) 151.36
b) 75.68
c) 24.32
d) 50.66
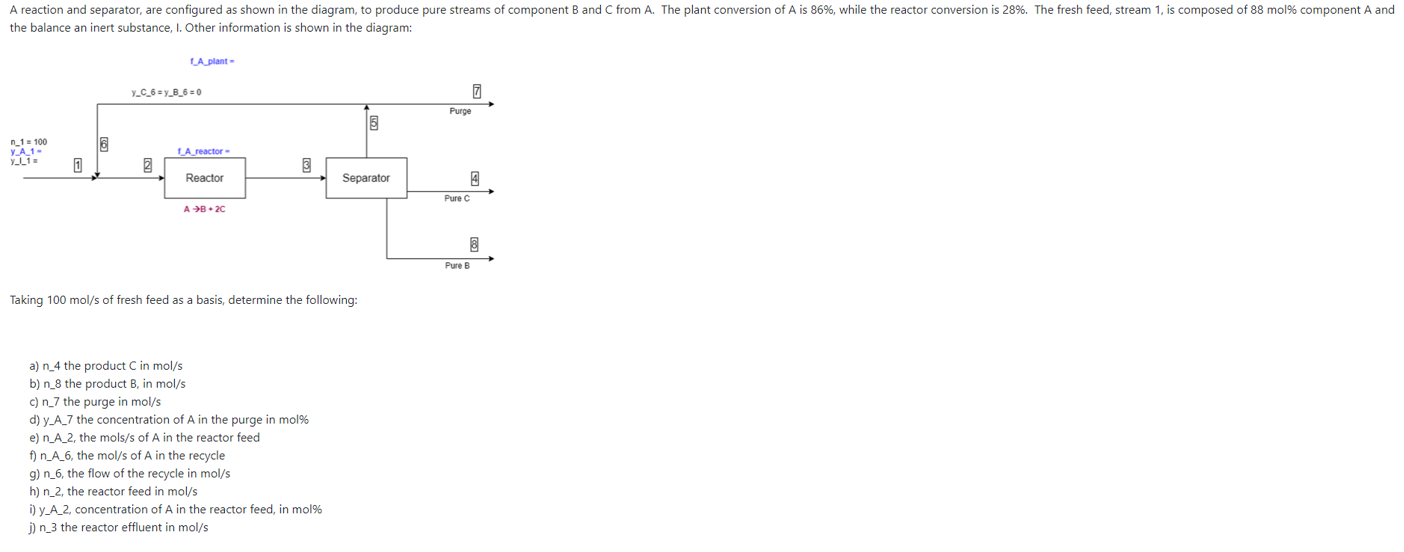
A reaction and separator, are configured as shown in the diagram, to produce pure streams of component B and C from A. The plant conversion of A is 86%, while the reactor conversion is 28%. The fresh feed, stream 1, is composed of 88 mol% component A and the balance an inert substance, I. Other information is shown in the diagram: LA plant- Y_C_6ny B 6=0 0 Purge 16 6 n_1 = 100 YA1- yli LA reactor- 0 2 Reactor Separator Pure C A B2C Pure B Taking 100 mol/s of fresh feed as a basis, determine the following: a) n_4 the product C in mol/s b) n_8 the product B, in mol/s c) n_7 the purge in mol/s d) y A_7 the concentration of A in the purge in mol% e) n_A_2, the mols/s of A in the reactor feed f) n_A_6, the mol/s of A in the recycle g) n_6, the flow of the recycle in mol/s h) n_2, the reactor feed in mol/s ) y_A_2, concentration of A in the reactor feed, in mol% j) n_3 the reactor effluent in mol/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


