Question: I have down the primary data from the lab. I'm just not exactly sure what the rest is asking/how to fill it out. I put
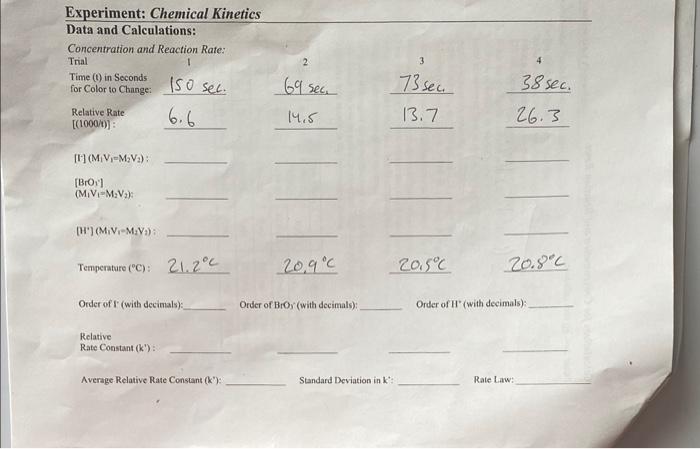
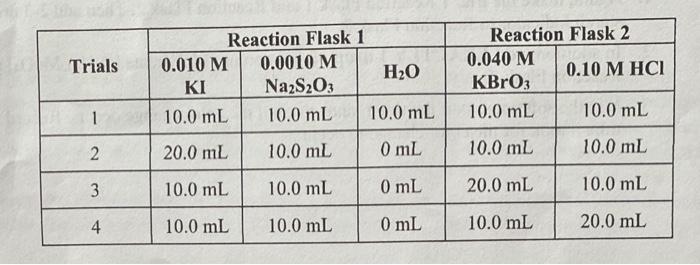

Data and Calculations: Concentration and Reaction Rate: Trial 6.669sec15.5213.773sec Time (i) in Seconds for Color to Change: Relative Rate [(1000/t)] : [II](M1V1=M2V2) : [Broy] (M1V1=M2V2): [H'] M1V1M2V2): Tempenture ( C):21.2C20.9C20.5C20.8C Order of F ( with decimals): Order of Bror'(with decimals): Order of H' (with decimals): Relative Rate Constant (k') : Average Relative Rate Constant (k) : Standard Deviation in k : Rate Law: \begin{tabular}{|c|c|c|c|c|c|} \hline \multirow{2}{*}{ Trials } & \multicolumn{3}{|c|}{ Reaction Flask 1 } & \multicolumn{2}{c|}{ Reaction Flask 2 } \\ \cline { 2 - 6 } & 0.010MKI & 0.0010MNa2S2O3 & H2O & 0.040MKBrO3 & 0.10MHCl \\ \hline 1 & 10.0mL & 10.0mL & 10.0mL & 10.0mL & 10.0mL \\ \hline 2 & 20.0mL & 10.0mL & 0mL & 10.0mL & 10.0mL \\ \hline 3 & 10.0mL & 10.0mL & 0mL & 20.0mL & 10.0mL \\ \hline 4 & 10.0mL & 10.0mL & 0mL & 10.0mL & 20.0mL \\ \hline \end{tabular} In this experiment you will investigate some of the main factors that influence chemical reaction rates: concentration, temperature and the action of catalysts. Using the data measured. you will be able to determine the rate law, the relative rate constant, and the activation energy of the reaction being studied. You will be investigating the reaction between iodide ion and bromate ion under acidic conditions. This reaction is rather slow at room temperature, however the rate will depend on the concentrations of iodide (I), bromate (BrO3), and acid (H+). 6I(aq)+BrO3(aq)+6H(aq)+3I2(aq)+Br(aq)+3H2O(l) One of the primary goals of this experiment is to determine the rate law. As we have learned, rate laws generally depend on the concentrations of the reactants in a given reaction. A generic rate law for the reaction is seen below. One of the goals of this lab is to determine the values of the exponents. rate=k[I]x[BrO3]y[H+]z In order to measure the above reaction, the iodine clock reaction will be used. The reaction below will be set up to run at the same time as the reaction above in the same solution. I2(aq)+2S2O3(aq)22I(aq)+S4O6(aq)2 Due to the iodine clock reaction (rection 3 ) being effectively instantaneous, the I2 produced in reaction 1 is immediately consumed until all S2O32 - has reacted. Once all the S2O32 - is consumed, the I2 from reaction 1 will remain in the solution and its concentration will increase. In the presence of starch, l2 turns the solution a blue color. In this lab, the same amount of S2O32 - is used in each trial, so the solution tuming blue tells us that a specific amount of S2O32 in reaction 3 has reacted. The concentration of S2O32 - is very small compared to the concentrations of the reactants in reaction 1 so we can assume the concentrations of I,BrO3, and H+remain unchanged throughout each trial, making the rate of each individual trial constant. However, the rates of each trial will be different from each other, because of the different initial concentrations of the reactants. For this system, we can express the rate of the reaction as a relative rate; a rate that can be compared to other trials of the same reaction with different reactant concentrations. By changing the concentrations of the reactants in reaction 1 and measuring the time it takes for the solution to turn blue we can calculate: 1) The relative rate of the reaction, 2) the exponents for each reactant in the rate law (the order with respect to each reactant), and 3) the relative rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


