Question: I have learned how to write the problem already, for instance. P=(0.39)^4 (1.7)^6 / R=(0.86)^4 (1.8)^5 My issue is in the actual calculation of the
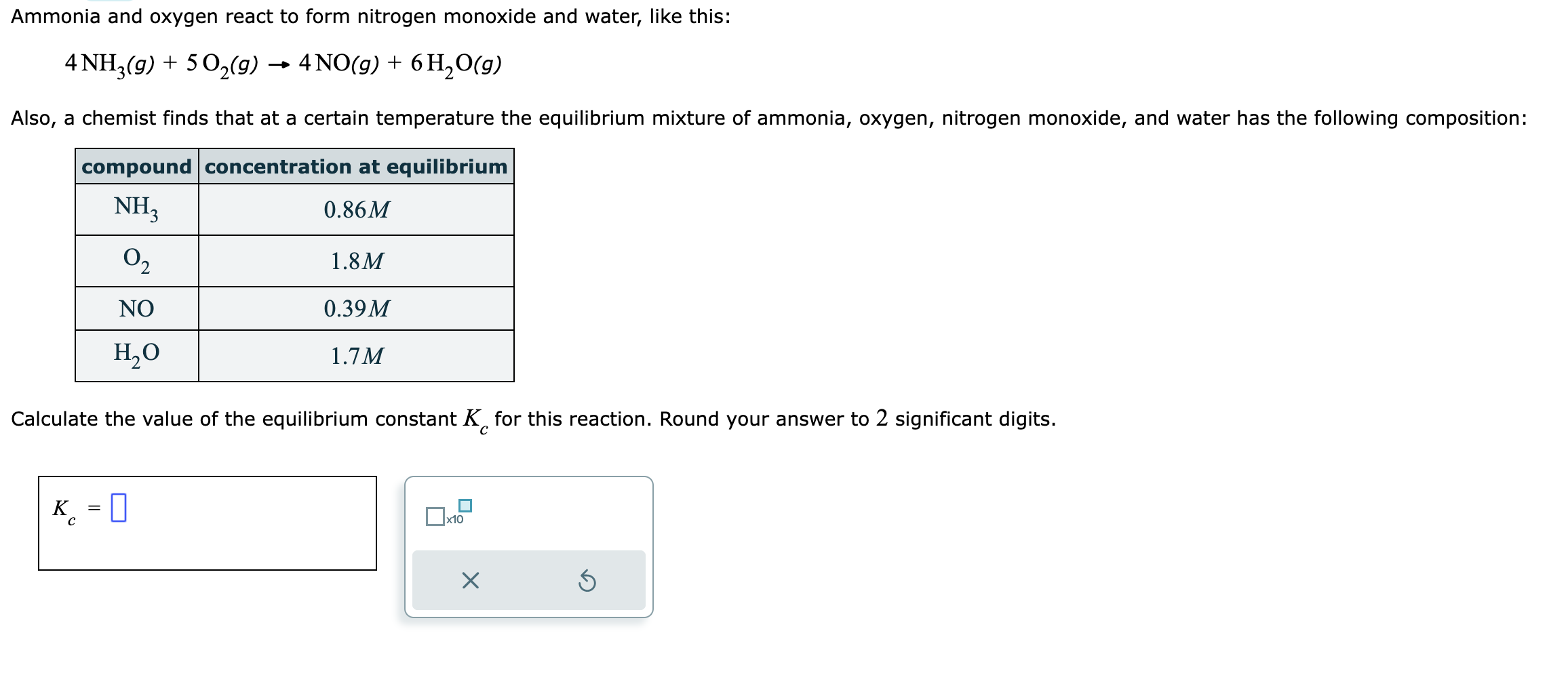
I have learned how to write the problem already, for instance.
P=(0.39)^4 (1.7)^6 / R=(0.86)^4 (1.8)^5
My issue is in the actual calculation of the problem. I am doing 0.39 x 4 x 1.7 x 6 % 0.86 x 4 x 1.8 x 5 but keep getting it wrong. Thanks in advance!
4NH3(g)+5O2(g)4NO(g)+6H2O(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


