Question: I have provided the question along with the chart it is wanting me to use. I dont quite understnd how to answer this problem. Please
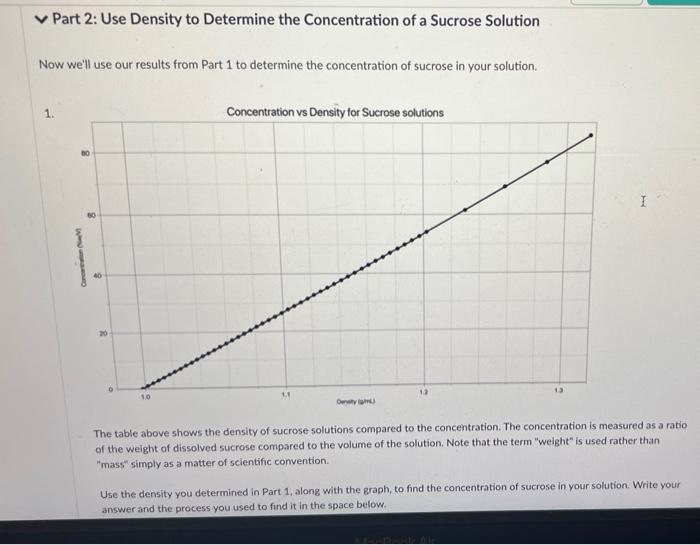
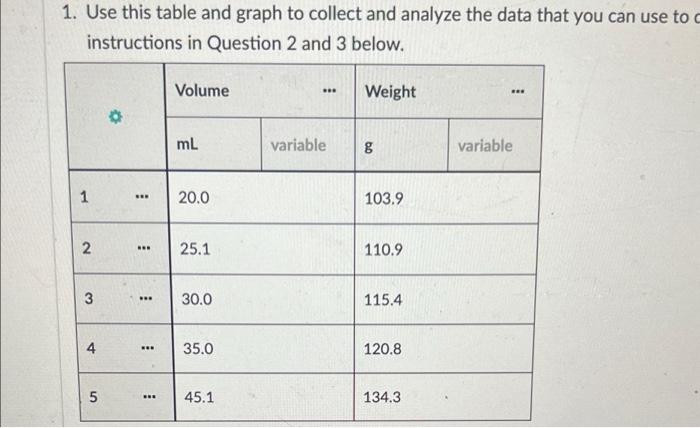
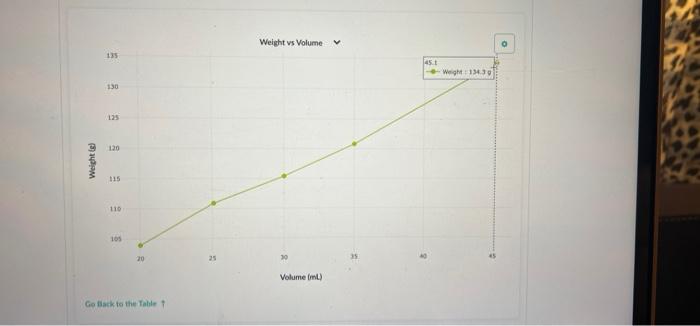
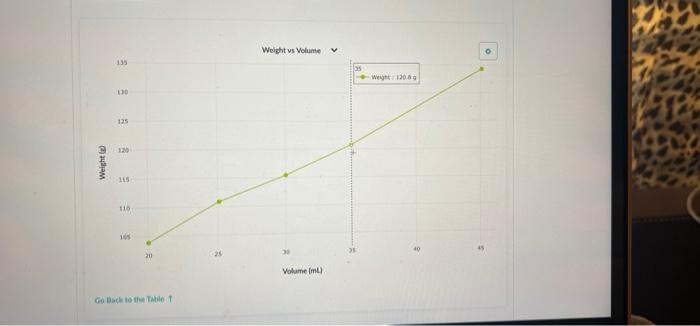
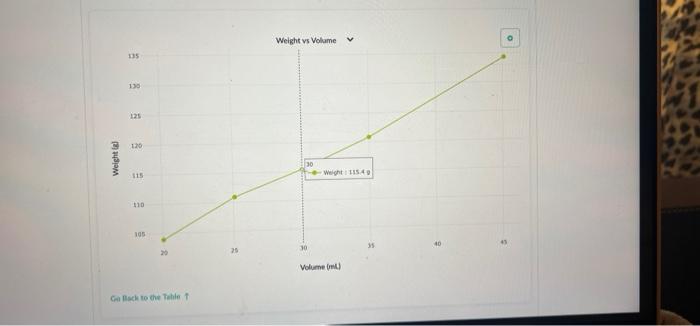
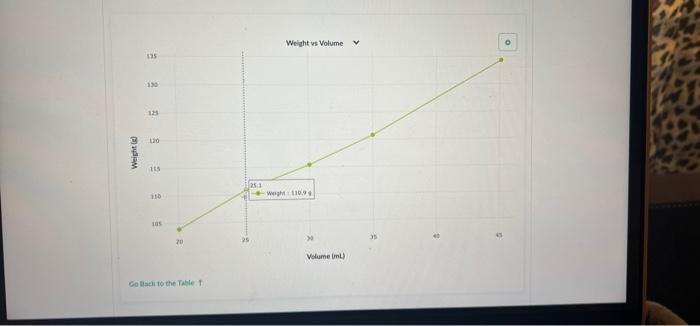
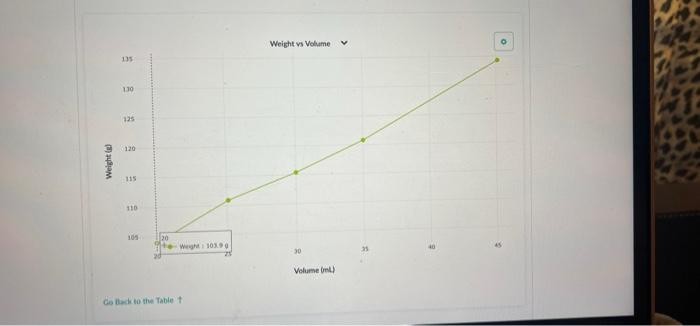
Part 2: Use Density to Determine the Concentration of a Sucrose Solution Now we'll use our results from Part 1 to determine the concentration of sucrose in your solution. 1. Concentration vs Density for Sucrose solutions I . 40 Care The table above shows the density of sucrose solutions compared to the concentration. The concentration is measured as a ratio of the weight of dissolved sucrose compared to the volume of the solution. Note that the term "weight" is used rather than "mass" simply as a matter of scientific convention Use the density you determined in Part 1, along with the graph, to find the concentration of sucrose in your solution. Write your answer and the process you used to find it in the space below. 1. Use this table and graph to collect and analyze the data that you can use to instructions in Question 2 and 3 below. Volume Weight BE 0 mL variable 8 variable 1 BB 20.0 103.9 2 25.1 110.9 3 30.0 115.4 4 . 35.0 120.8 5 888 45.1 134.3 Weight vs Volume V 135 45.0 -- WM13439 130 125 120 Weight is 115 110 105 20 30 Volument) Go Back to the Table Weight vs Volunte 135 Wet 120.89 130 135 120 Weight LIS 11 $ 20 Volume mu Go Back to the Tablet Weight vs Volume 135 130 125 120 Weights 115 Weight:11549 110 105 40 30 20 Volume mu Galack to the Table 1 Weight vs Volume V O 135 130 123 110 Weight 115 253 110 W 110.00 105 Volume mi) Go Back to the Tablet Weight vs Volume v 135 130 135 120 Weight SIL 110 03 120 W 103.90 OC Volument) Cack to the Tablet Part 2: Use Density to Determine the Concentration of a Sucrose Solution Now we'll use our results from Part 1 to determine the concentration of sucrose in your solution. 1. Concentration vs Density for Sucrose solutions I . 40 Care The table above shows the density of sucrose solutions compared to the concentration. The concentration is measured as a ratio of the weight of dissolved sucrose compared to the volume of the solution. Note that the term "weight" is used rather than "mass" simply as a matter of scientific convention Use the density you determined in Part 1, along with the graph, to find the concentration of sucrose in your solution. Write your answer and the process you used to find it in the space below. 1. Use this table and graph to collect and analyze the data that you can use to instructions in Question 2 and 3 below. Volume Weight BE 0 mL variable 8 variable 1 BB 20.0 103.9 2 25.1 110.9 3 30.0 115.4 4 . 35.0 120.8 5 888 45.1 134.3 Weight vs Volume V 135 45.0 -- WM13439 130 125 120 Weight is 115 110 105 20 30 Volument) Go Back to the Table Weight vs Volunte 135 Wet 120.89 130 135 120 Weight LIS 11 $ 20 Volume mu Go Back to the Tablet Weight vs Volume 135 130 125 120 Weights 115 Weight:11549 110 105 40 30 20 Volume mu Galack to the Table 1 Weight vs Volume V O 135 130 123 110 Weight 115 253 110 W 110.00 105 Volume mi) Go Back to the Tablet Weight vs Volume v 135 130 135 120 Weight SIL 110 03 120 W 103.90 OC Volument) Cack to the Tablet
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


