Question: I have tried this so many ways and cannot figure out how fo solve. Please help!!! 17.45 For the following reaction, Kp=6.5104 at 308K :
I have tried this so many ways and cannot figure out how fo solve. Please help!!! 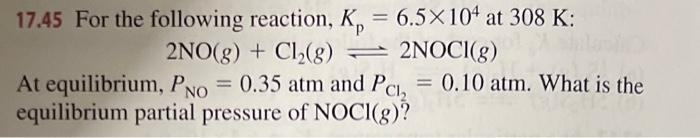
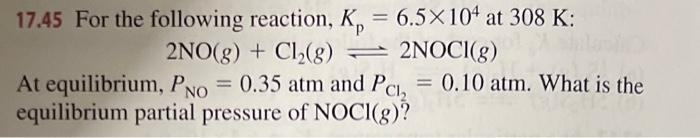
17.45 For the following reaction, Kp=6.5104 at 308K : 2NO(g)+Cl2(g)2NOCl(g) At equilibrium, PNO=0.35atm and PCl2=0.10atm. What is the equilibrium partial pressure of NOCl(g)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


