Question: I JUST NEED PART C Problem 1 Aluminum is a common metal used to make anything from beverage cans to bicycles to fighter jets. One
I JUST NEED PART "C"
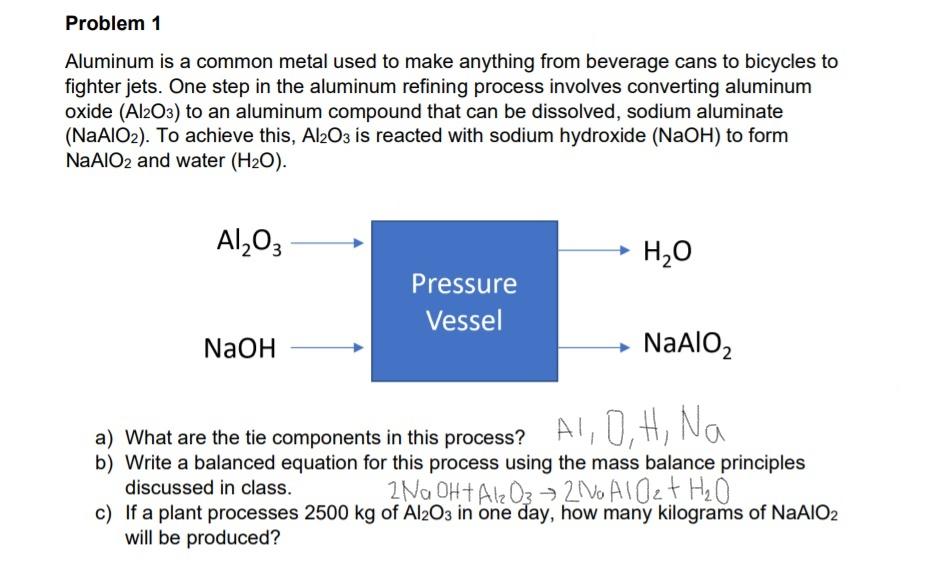
Problem 1 Aluminum is a common metal used to make anything from beverage cans to bicycles to fighter jets. One step in the aluminum refining process involves converting aluminum oxide (Al2O3) to an aluminum compound that can be dissolved, sodium aluminate (NaAlO2). To achieve this, Al2O3 is reacted with sodium hydroxide (NaOH) to form NaAlO2 and water (H2O). Al2O3 H2O Pressure Vessel NaOH NaAlO2 a) What are the tie components in this process? Al, O, H, Na ? b) Write a balanced equation for this process using the mass balance principles discussed in class. Z Na OHT Al2 O 210 AIO 2t HO c) If a plant processes 2500 kg of Al2O3 in one day, how many kilograms of NaAlO2 will be produced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


