Question: i just need the answer to the third question. thank you At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is
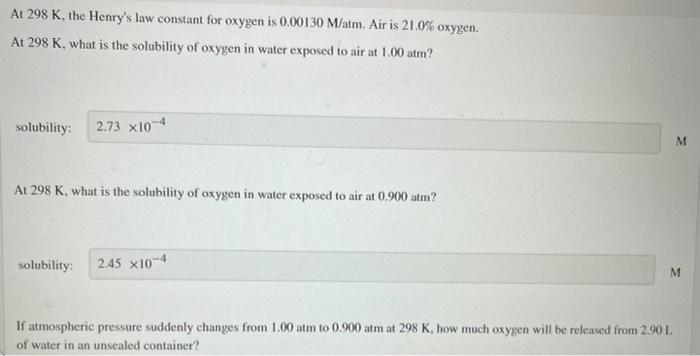
At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is 21.0% oxygen. At 298 K, what is the solubility of oxygen in water exposed to air at 1.00 atm? solubility: 2.73 x10-4 M M At 298 K, what is the solubility of oxygen in water exposed to air at 0.900 atm? solubility: 2.45 x10-4 M If atmospheric pressure suddenly changes from 1.00 atm to 0.900 atm at 298 K, how much oxygen will be released from 2.90 L of water in an unsealed container
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


