Question: I keep getting a negative exponent for y. I think i might have messed up the calculation for the Rate in table 2 1. Using
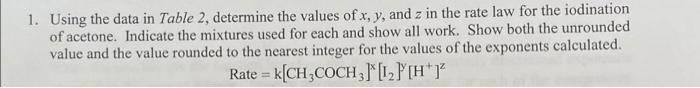
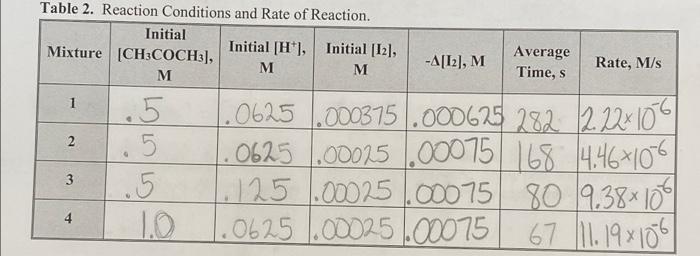
1. Using the data in Table 2, determine the values of x, y, and z in the rate law for the iodination of acetone. Indicate the mixtures used for each and show all work. Show both the unrounded value and the value rounded to the nearest integer for the values of the exponents calculated. Rate = k[CH,COCH3)*12 [H"]? Table 2. Reaction Conditions and Rate of Reaction. Initial Mixture [CH3COCH3], Initial [H*], Initial [12], M M M -A[12], M Average Time, s Rate, M/s 1 -6 2 2 .0625 .000375.000625 282 2222106 .0625 .00025 .00075 168 14.4671096 1.125 1.00025 .00075 80 19.38100 1.0625 .00025.00075 67 11.19x106 .5 . .5 10 3 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


