Question: i keep getting it wrong please make sure its right A mixture of 0.04096mol of C2H6,0.05994mol of N2,0.04520mol of NH3, and 0.02898mol of C2H4 is
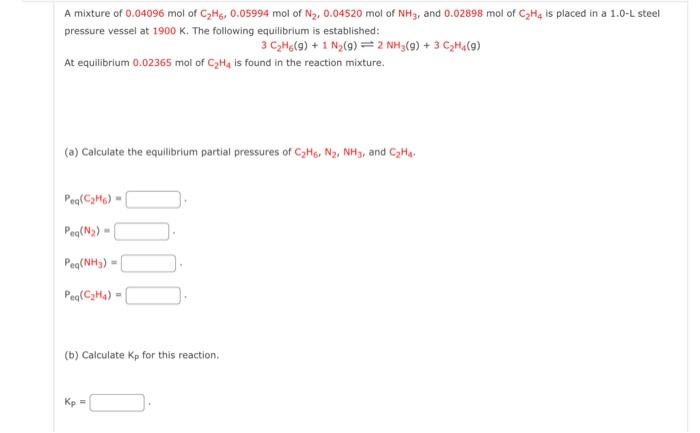
A mixture of 0.04096mol of C2H6,0.05994mol of N2,0.04520mol of NH3, and 0.02898mol of C2H4 is placed in a 1.0L steel pressure vessel at 1900K. The following equilibrium is established: 3C2H6(g)+1N2(g)2NH3(g)+3C2H4(g) At equilibrium 0.02365mol of C2H4 is found in the reaction mixture. (a) Calculate the equilibrium partial pressures of C2H6,N2,NH3, and C2H4. Peq(C2H6)= Peq(N2)= Peqeq(NH3)= Peq(C2H4)= (b) Calculate Kp for this reaction. Kp=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


