Question: I keep getting this problem wrong. Any help? Its for a python programming class. This is the data file needed for this problem. The viscosity
I keep getting this problem wrong. Any help? Its for a python programming class.
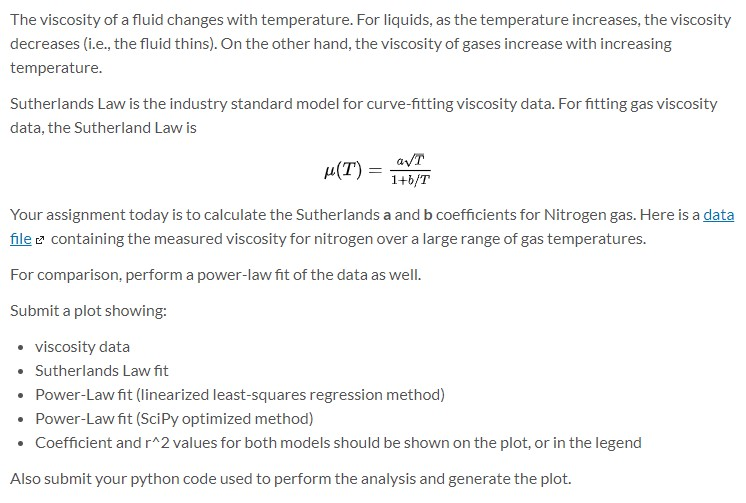
This is the data file needed for this problem.
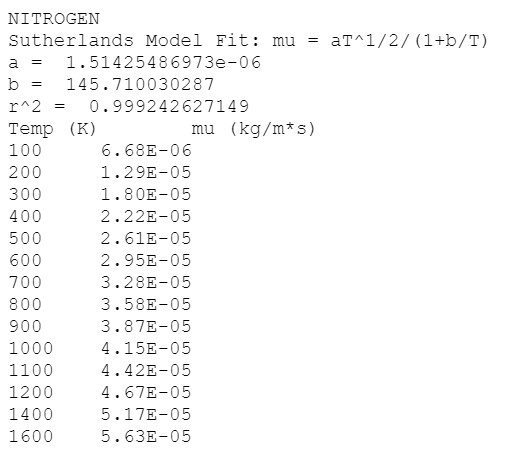
The viscosity of a fluid changes with temperature. For liquids, as the temperature increases, the viscosity decreases (i.e., the fluid thins). On the other hand, the viscosity of gases increase with increasing temperature. Sutherlands Law is the industry standard model for curve-fitting viscosity data. For fitting gas viscosity data, the Sutherland Law is *(T) = av Your assignment today is to calculate the Sutherlands a and b coefficients for Nitrogen gas. Here is a data file e containing the measured viscosity for nitrogen over a large range of gas temperatures. For comparison, perform a power-law fit of the data as well. Submit a plot showing: viscosity data Sutherlands Law fit Power-Law fit linearized least-squares regression method) Power-Law fit (SciPy optimized method) Coefficient and r^2 values for both models should be shown on the plot, or in the legend Also submit your python code used to perform the analysis and generate the plot. NITROGEN Sutherlands Model Fit: mu = aT^1/2/(1+b/T) a = 1.51425486973e-06 b = 145.710030287 r^2 = 0.999242627149 Temp (K) mu (kg/m*s) 100 6.68E-06 200 1.29E-05 300 1.80E-05 400 2.22E-05 500 2.61E-05 600 2.95E-05 700 3.28E-05 800 3.58E-05 900 3.87E-05 1000 4.15E-05 1100 4.42E-05 1200 4.67E-05 1400 5.17E-05 1600 5.63E-05 The viscosity of a fluid changes with temperature. For liquids, as the temperature increases, the viscosity decreases (i.e., the fluid thins). On the other hand, the viscosity of gases increase with increasing temperature. Sutherlands Law is the industry standard model for curve-fitting viscosity data. For fitting gas viscosity data, the Sutherland Law is *(T) = av Your assignment today is to calculate the Sutherlands a and b coefficients for Nitrogen gas. Here is a data file e containing the measured viscosity for nitrogen over a large range of gas temperatures. For comparison, perform a power-law fit of the data as well. Submit a plot showing: viscosity data Sutherlands Law fit Power-Law fit linearized least-squares regression method) Power-Law fit (SciPy optimized method) Coefficient and r^2 values for both models should be shown on the plot, or in the legend Also submit your python code used to perform the analysis and generate the plot. NITROGEN Sutherlands Model Fit: mu = aT^1/2/(1+b/T) a = 1.51425486973e-06 b = 145.710030287 r^2 = 0.999242627149 Temp (K) mu (kg/m*s) 100 6.68E-06 200 1.29E-05 300 1.80E-05 400 2.22E-05 500 2.61E-05 600 2.95E-05 700 3.28E-05 800 3.58E-05 900 3.87E-05 1000 4.15E-05 1100 4.42E-05 1200 4.67E-05 1400 5.17E-05 1600 5.63E-05
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


