Question: I know it's an ether. unknown is anisole Post-Lab Questions Include copies of your IR spectrum, GC/MS chromatogram and mass spectrum, and H NMR spectra
I know it's an ether. unknown is anisole

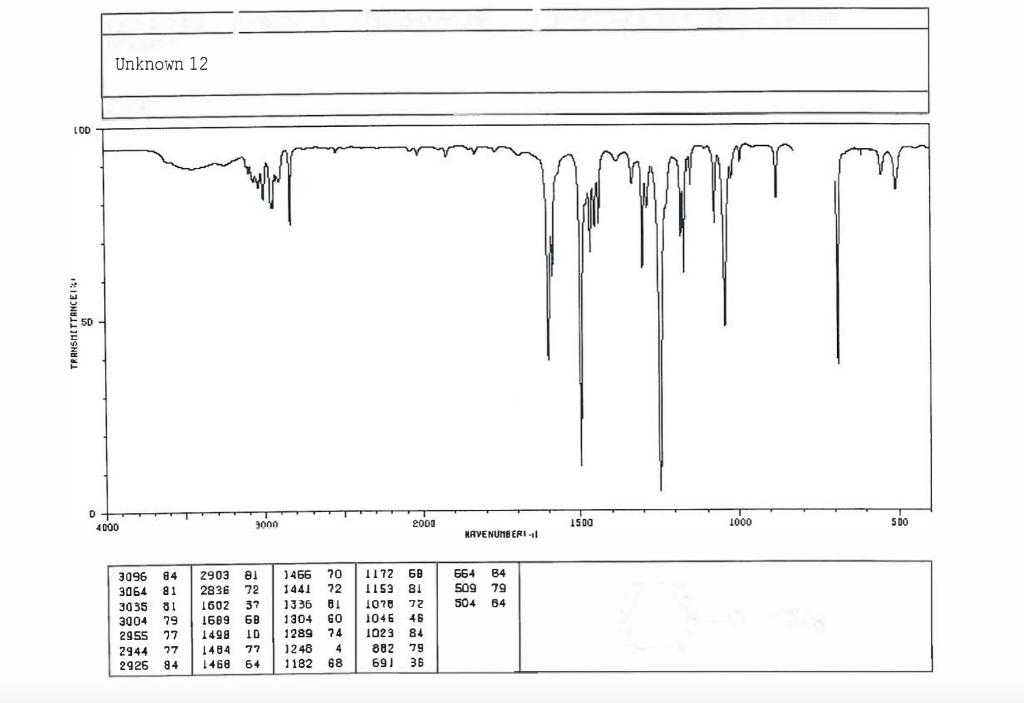
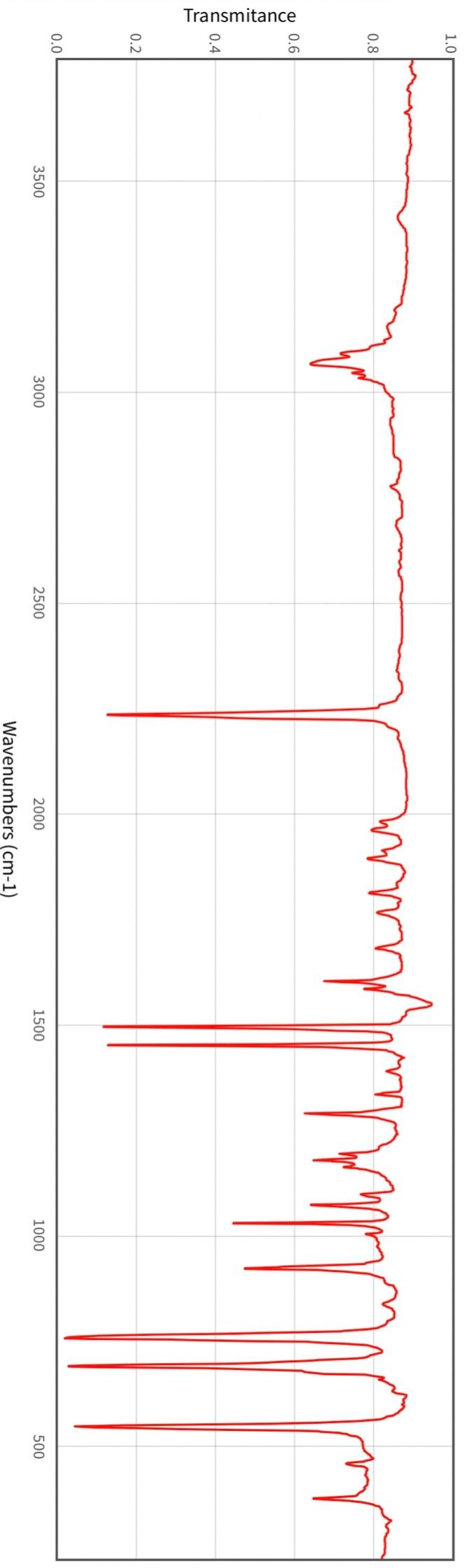
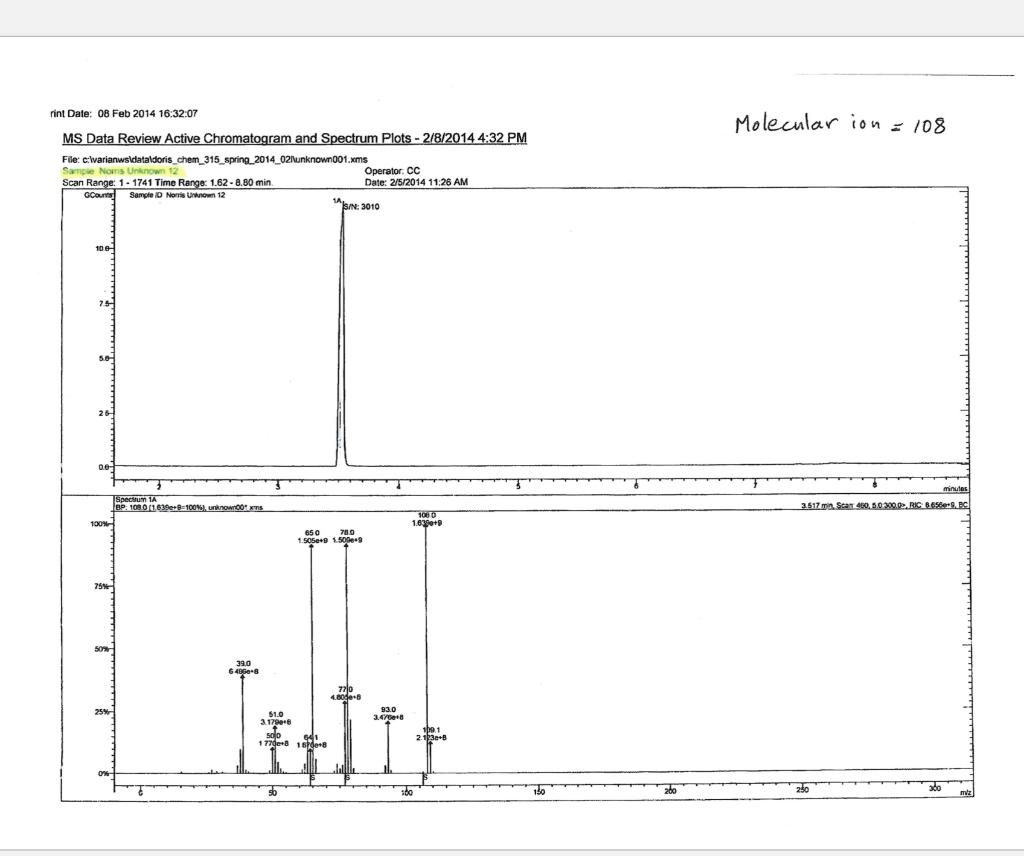
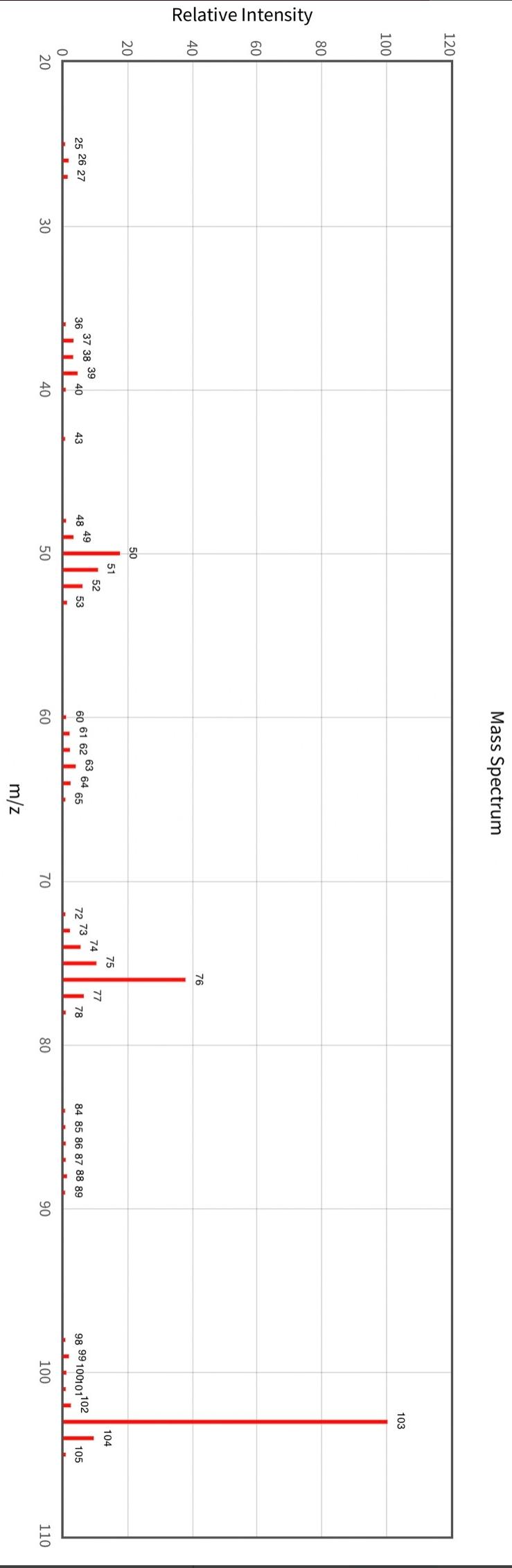
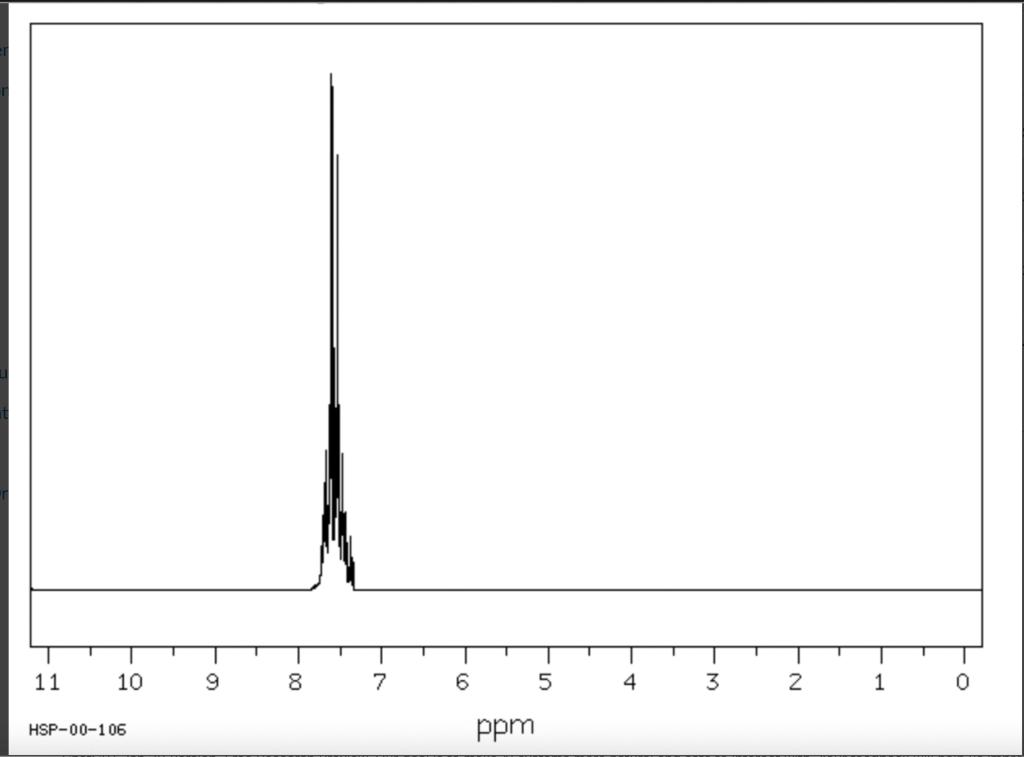
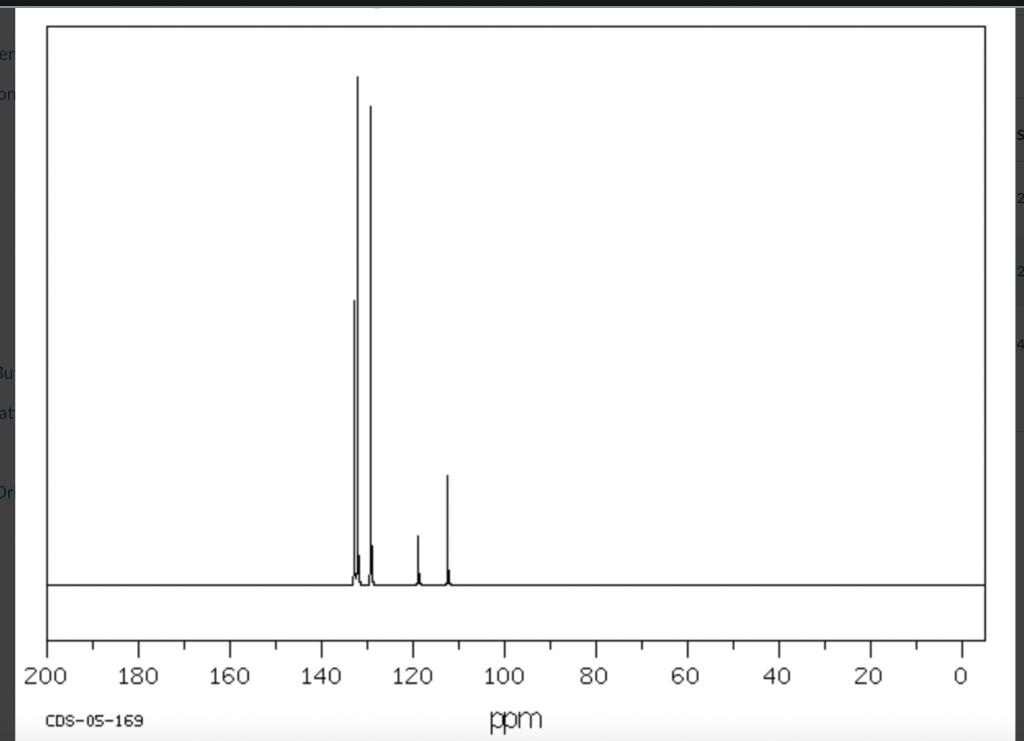
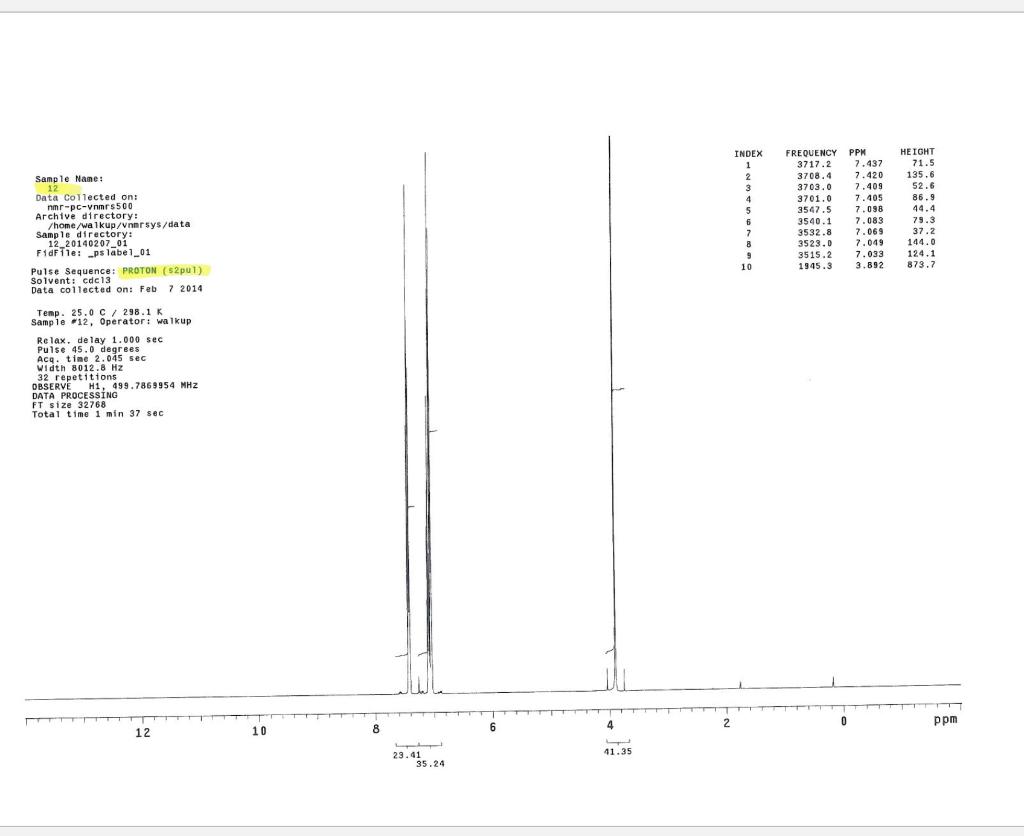
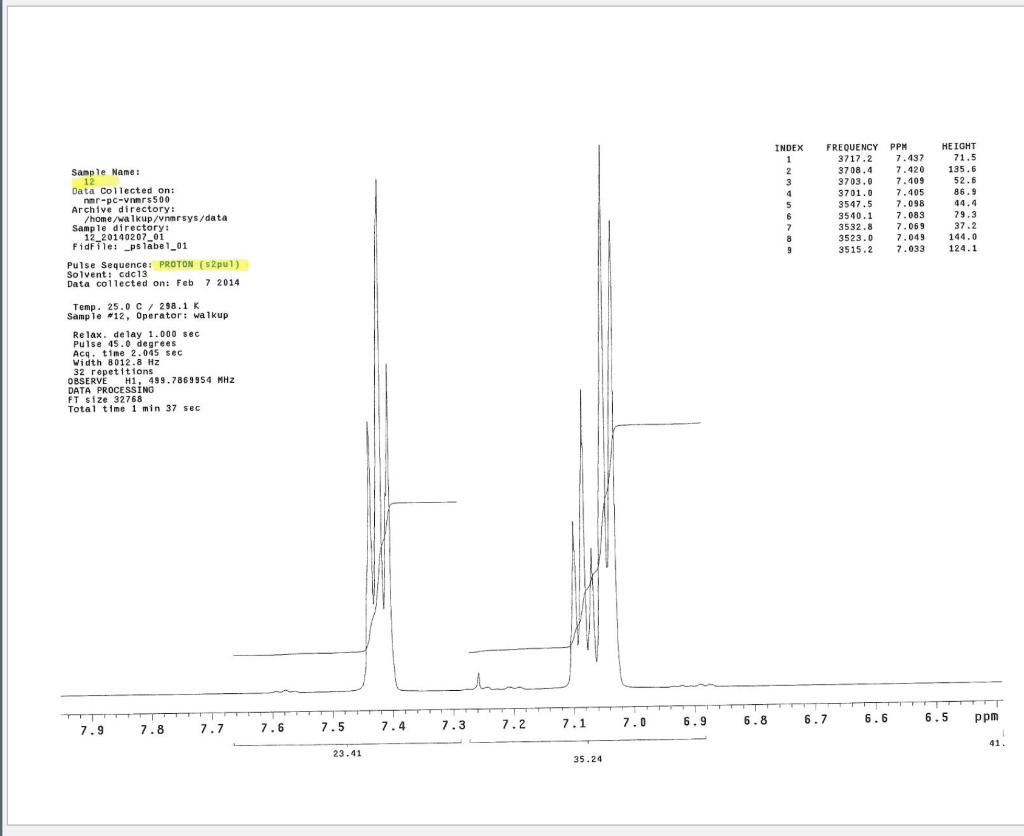
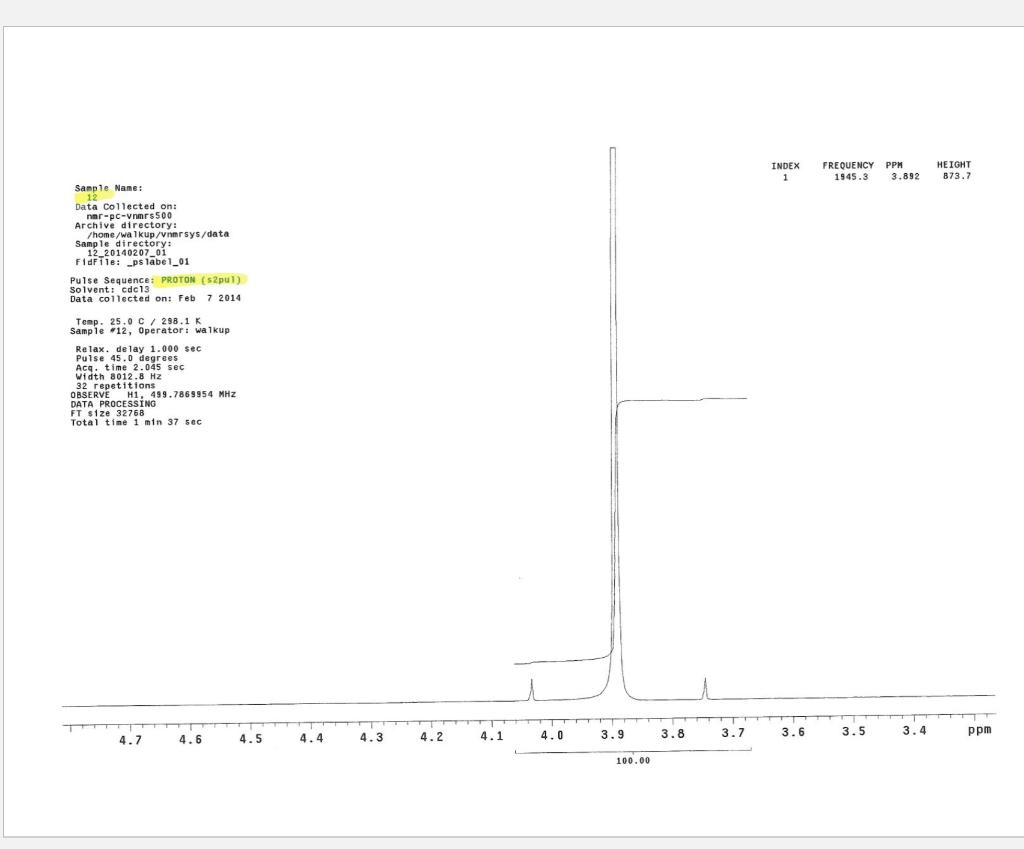
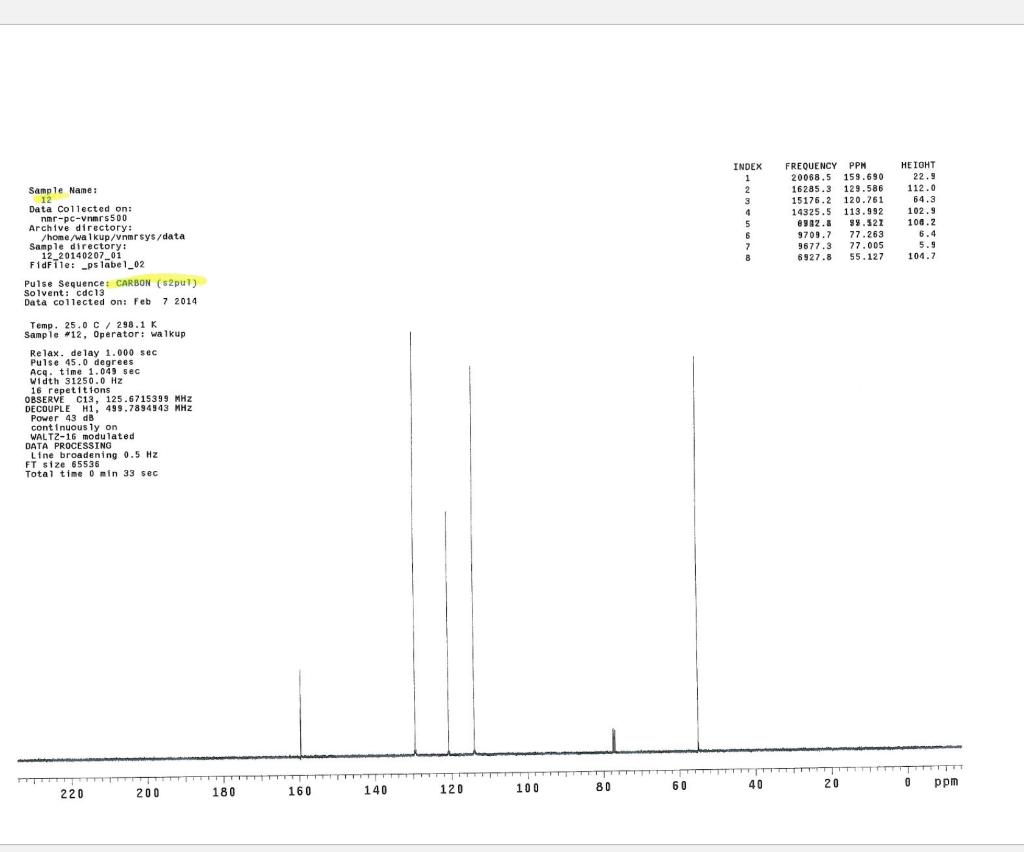
Post-Lab Questions Include copies of your IR spectrum, GC/MS chromatogram and mass spectrum, and H NMR spectra with your lab report. Make sure that you identify the label of your unknown in your report. (1) Propose a structure for your unknown that is consistent with the IR, GC/MS, and ' 1H and 13C NMR spectra. (Note: also the density, if requested by your instructor) (2) What are the major peaks in your IR spectrum. Assign these peaks to specific bond motions for functional groups that are present in your unknown. Note: only assign the major peaks in the 15004000cm1 region. Absorptions in the 600 to 1200cm1 region can be extremely helpful in determining how substituants are distributed on the arene ring. If you are interested in interpreting this region of your IR spectrum, consult your laboratory instructor. (3) What is the m/z value for the molecular ion for your unknown? What is the m/z value for the base peak in your unknown? Provide possible structures for ions that could be responsible for the major fragments (>25% intensity) in your mass spectrum. (4) Assign the peaks in your 1HNMR spectrum to the protons in your proposed structure. Explain how the chemical shifts, integrals and coupling in your spectrum are consistent with the proposed structure. (5) Assign the peaks in your 13C NMR spectrum to the carbons in your proposed structure. Explain how the chemical shifts in the spectrum are consistent with the proposed structure. Unknown 12 \begin{tabular}{|ll|ll|lr|ll|ll|l|} \hline 3096 & 84 & 2903 & 81 & 1466 & 70 & 1172 & 68 & 664 & 84 & \\ 3064 & 81 & 2836 & 72 & 1441 & 72 & 1153 & 81 & 509 & 79 & \\ 3035 & 81 & 1602 & 37 & 1336 & 81 & 1078 & 72 & 504 & 84 & \\ 3004 & 79 & 1689 & 68 & 1304 & 60 & 1046 & 46 & & & \\ 2955 & 77 & 1498 & 10 & 1289 & 74 & 1023 & 84 & & \\ 2944 & 77 & 1484 & 77 & 1248 & 4 & 882 & 79 & & \\ 2926 & 84 & 1468 & 64 & 1182 & 68 & 691 & 36 & & & \\ \hline \end{tabular} rint Date: 08Feb2014 16:32:07 Molecular ion =108 MS Data Review Active Chromatogram and Spectrum Plots - 2/8/2014 4:32 PM File: c:warianwsldataldoris_chem_315_spring_2014_02hunknown001.wms Scan Range: 1- 1741 Time Range: 1.628.80min. Operator, CC Relative Intensity Sample Nane: Data Collected on: nar-pc-vnmes 500 Archive directory: /home/wa 1kup/ /vnmrsys / data Sample directory: FidFi1e: _ps iabel_o1 Puise Sequence: PRotok (sipui) Solvent: cde13. Data co1lected on: Feb 72014 Tcop25,0c/288,1K Sample 12 , 0perator: wa.kup Relax. delay 1.000sec Pulse 45, 0 thegrees Acq, time 2.045 32 repetitions OBSERVE H1, 499.7869954 MHz DATA PROCESSING FT s12e 32768 Total time 1 inth 37sec Samp 1 e Name: Data Co11ected on: nmr-pc-vnmr 550 Archive difectory: /home/wa. 1kup/ vnmrsys / data Sample direrctory: 122014020701 F1dF11e:=951abe1_02 Pulse Sequencef CARBON (s2pul) Solvent: cdc 13 7ln1A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


