Question: i know this is completely wrong and im stumped on it :/ Balance the following net ionic reaction in basic conditions. ClO+CrO2Cl2+CrO42 If a coefficient
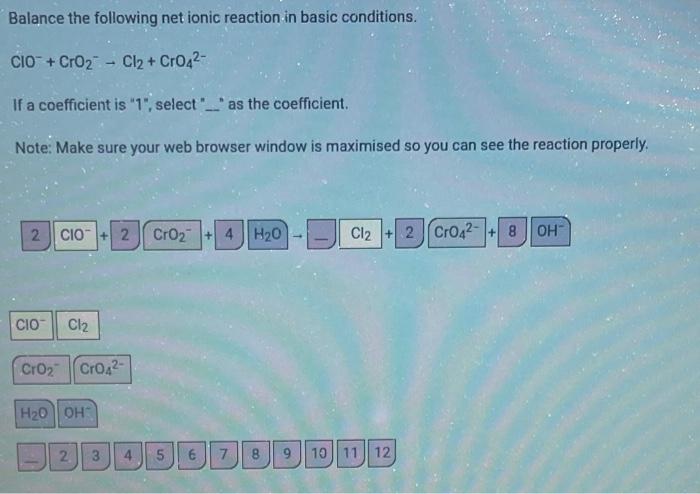
Balance the following net ionic reaction in basic conditions. ClO+CrO2Cl2+CrO42 If a coefficient is " 1 ", select ". " as the coefficient. Note: Make sure your web browser window is maximised so you can see the reaction properly. 2.ClO+2+CrO2+4+H2OCl2+2 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


