Question: I know you can't solve it without the graphs but can you just explain how to go about doing it a (30 pts) Tetrahydrofuran (THF)
I know you can't solve it without the graphs but can you just explain how to go about doing it
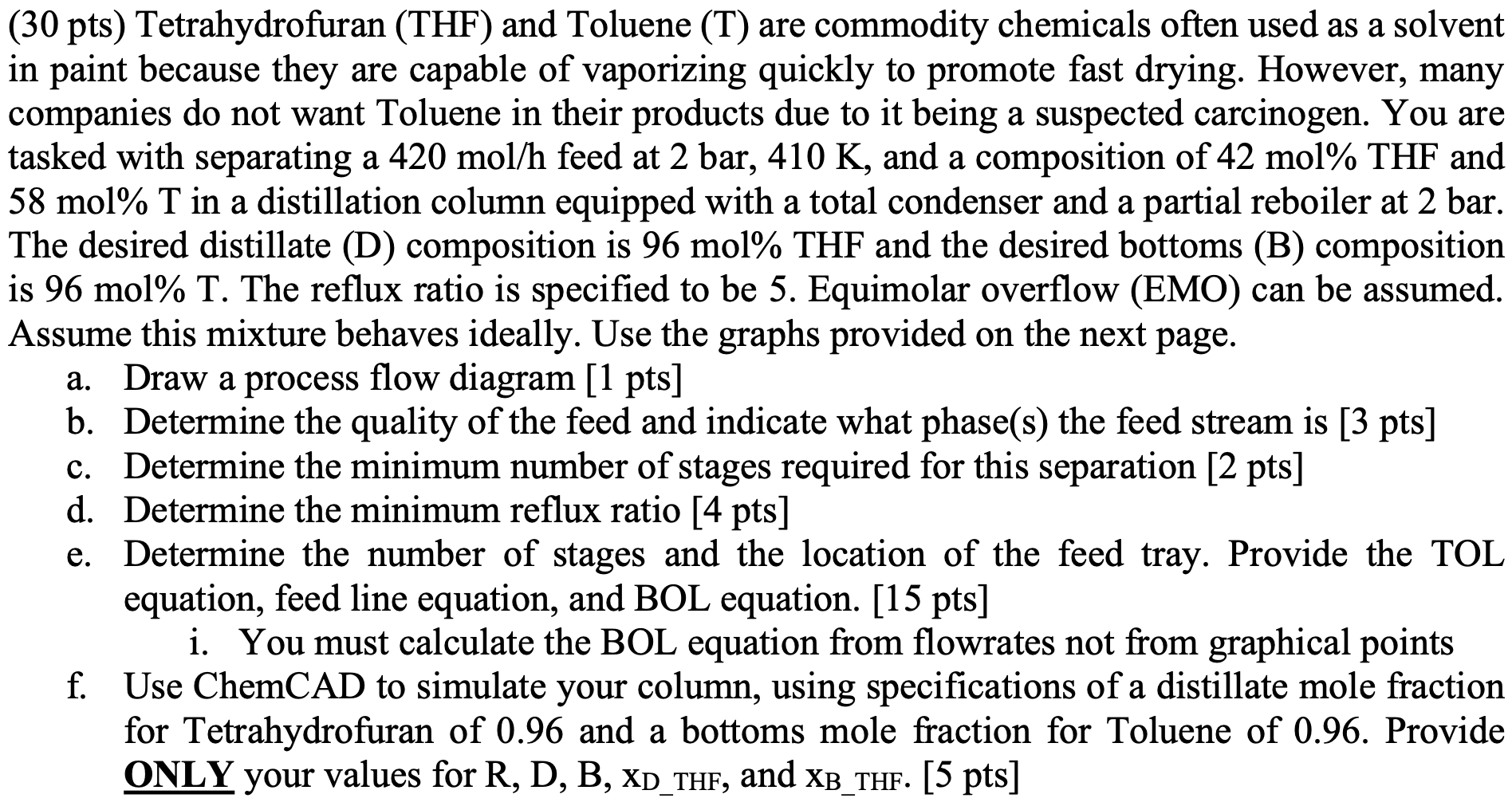
a (30 pts) Tetrahydrofuran (THF) and Toluene (T) are commodity chemicals often used as a solvent in paint because they are capable of vaporizing quickly to promote fast drying. However, many companies do not want Toluene in their products due to it being a suspected carcinogen. You are tasked with separating a 420 mol/h feed at 2 bar, 410 K, and a composition of 42 mol% THF and 58 mol% T in a distillation column equipped with a total condenser and a partial reboiler at 2 bar. The desired distillate (D) composition is 96 mol% THF and the desired bottoms (B) composition is 96 mol% T. The reflux ratio is specified to be 5. Equimolar overflow (EMO) can be assumed. Assume this mixture behaves ideally. Use the graphs provided on the next page. a. Draw a process flow diagram [1 pts] b. Determine the quality of the feed and indicate what phase(s) the feed stream is [3 pts] c. Determine the minimum number of stages required for this separation [2 pts] d. Determine the minimum reflux ratio [4 pts] e. Determine the number of stages and the location of the feed tray. Provide the TOL equation, feed line equation, and BOL equation. [15 pts] i. You must calculate the BOL equation from flowrates not from graphical points f. Use ChemCAD to simulate your column, using specifications of a distillate mole fraction for Tetrahydrofuran of 0.96 and a bottoms mole fraction for Toluene of 0.96. Provide ONLY your values for R, D, B, XD_THF, and XB_THF. [5 pts]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


