Question: i need a detailed working for these problems and explanations That is the all of the information i have unfortunately . May you please work
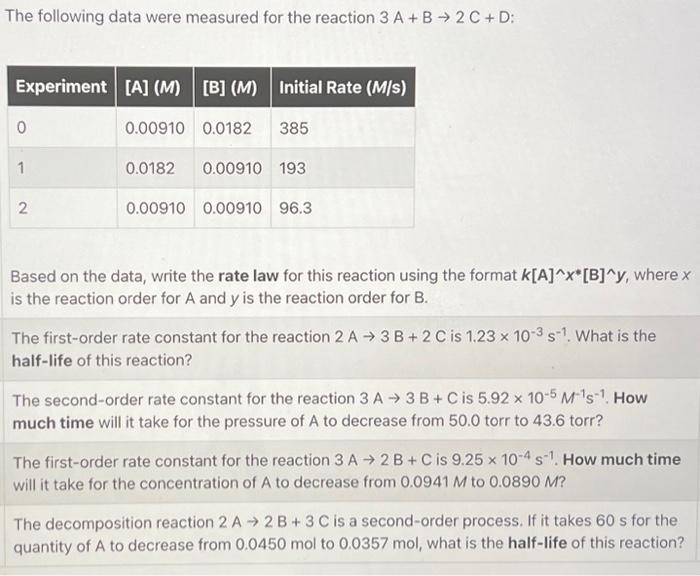
The following data were measured for the reaction 3A+B2C+D : Based on the data, write the rate law for this reaction using the format k[A]x[B]y, where x is the reaction order for A and y is the reaction order for B. The first-order rate constant for the reaction 2A3B+2C is 1.23103s1. What is the half-life of this reaction? The second-order rate constant for the reaction 3A3B+C is 5.92105M1s1. How much time will it take for the pressure of A to decrease from 50.0 torr to 43.6 torr? The first-order rate constant for the reaction 3A2B+C is 9.25104s1. How much time will it take for the concentration of A to decrease from 0.0941M to 0.0890M ? The decomposition reaction 2A2B+3C is a second-order process. If it takes 60s for the quantity of A to decrease from 0.0450mol to 0.0357mol, what is the half-life of this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


