Question: I need a step by step answer of question 1 and 2 and an explanation of how you got those results. Please, I will leave
I need a step by step answer of question and and an explanation of how you got those results. Please, I will leave a like.
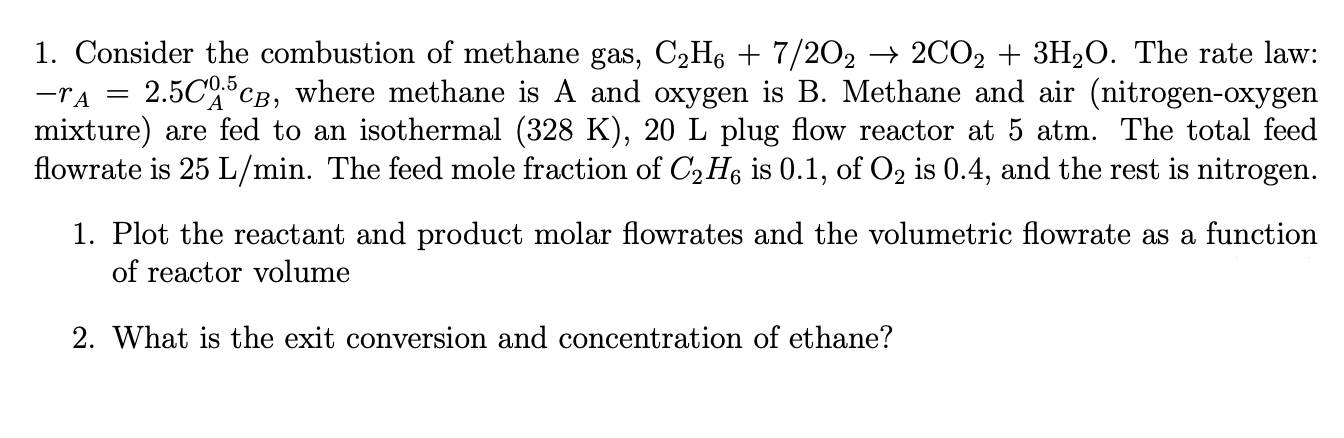
Consider the combustion of methane gas, The rate law:
where methane is A and oxygen is B Methane and air nitrogenoxygen
mixture are fed to an isothermal plug flow reactor at atm. The total feed
flowrate is The feed mole fraction of is of is and the rest is nitrogen.
Plot the reactant and product molar flowrates and the volumetric flowrate as a function
of reactor volume
What is the exit conversion and concentration of ethane?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


