Question: I need an expert help to answer this question correctly. Please help me by giving the right answer. please skip if you don't know the
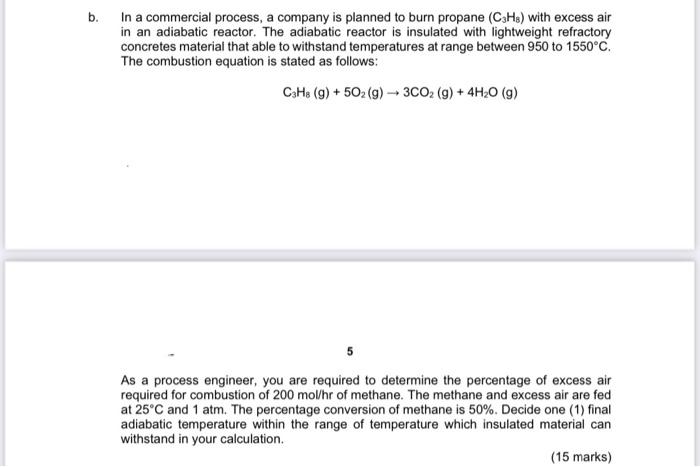
b. In a commercial process, a company is planned to burn propane (CzHs) with excess air in an adiabatic reactor. The adiabatic reactor is insulated with lightweight refractory concretes material that able to withstand temperatures at range between 950 to 1550C. The combustion equation is stated as follows: C3H8 (9) +502 (g) + 3CO2 (9) + 4H20 (9) As a process engineer, you are required to determine the percentage of excess air required for combustion of 200 mol/hr of methane. The methane and excess air are fed at 25C and 1 atm. The percentage conversion of methane is 50%. Decide one (1) final adiabatic temperature within the range of temperature which insulated material can withstand in your calculation. (15 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


