Question: i need clear answer pls The irreversible elementary gas phase reaction 2A B+C Is carried out in a packed bed reactor containing 100 kg of
i need clear answer pls
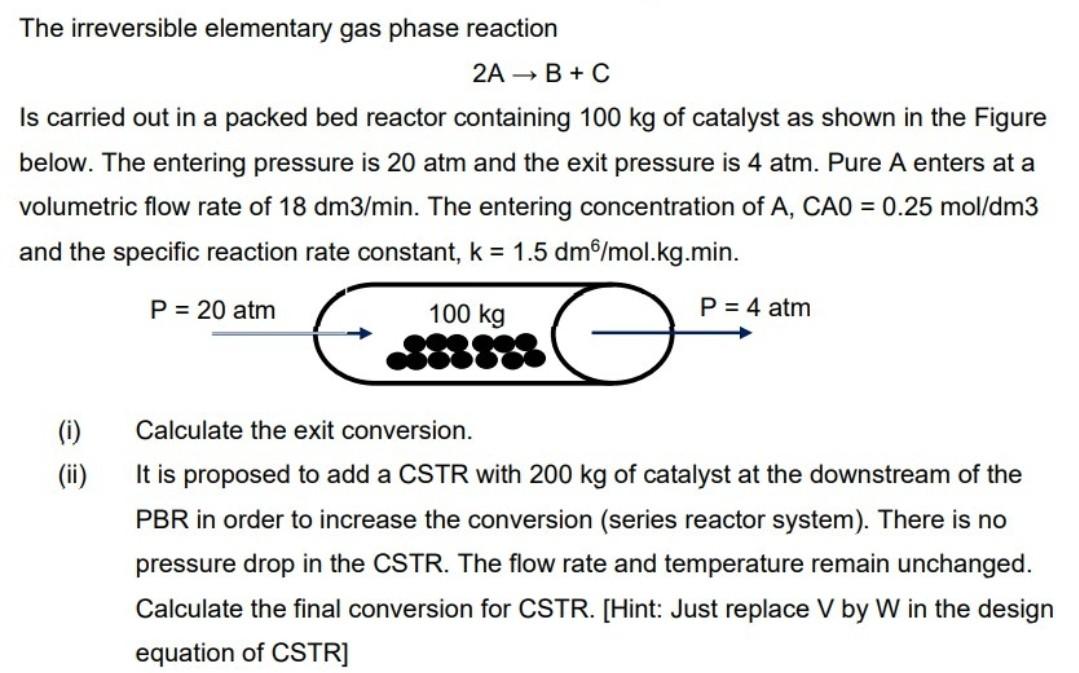
The irreversible elementary gas phase reaction 2A B+C Is carried out in a packed bed reactor containing 100 kg of catalyst as shown in the Figure below. The entering pressure is 20 atm and the exit pressure is 4 atm. Pure A enters at a volumetric flow rate of 18 dm3/min. The entering concentration of A, CAO = 0.25 mol/dm3 and the specific reaction rate constant, k = 1.5 dm/mol.kg.min. P = 20 atm 100 kg P = 4 atm (i) (ii) Calculate the exit conversion. It is proposed to add a CSTR with 200 kg of catalyst at the downstream of the PBR in order to increase the conversion (series reactor system). There is no pressure drop in the CSTR. The flow rate and temperature remain unchanged. Calculate the final conversion for CSTR. [Hint: Just replace V by W in the design equation of CSTR]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


