Question: i need clear solving for A and B ki A Dkz, U Pure A is fed to a 1.0-dm CSTR where it reacts to form
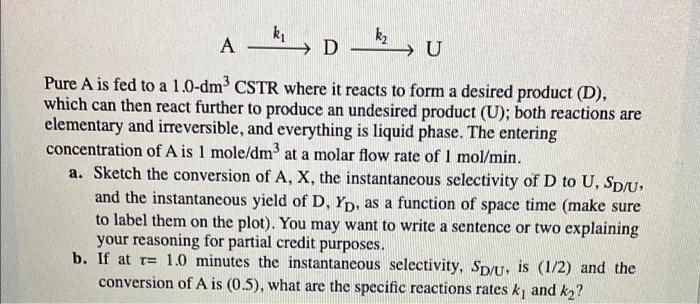
ki A Dkz, U Pure A is fed to a 1.0-dm CSTR where it reacts to form a desired product (D), which can then react further to produce an undesired product (U); both reactions are elementary and irreversible, and everything is liquid phase. The entering concentration of A is 1 mole/dm at a molar flow rate of 1 mol/min. a. Sketch the conversion of A, X, the instantaneous selectivity of D to U, SD/U, and the instantaneous yield of D, Yp, as a function of space time (make sure to label them on the plot). You may want to write a sentence or two explaining your reasoning for partial credit purposes. b. If at t= 1.0 minutes the instantaneous selectivity, Sp/u, is (1/2) and the conversion of A is (0.5), what are the specific reactions rates ky and k2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


