Question: i need help 4. Weigh a small, dean, dry beaker and set it aside. Pour the solution into the 150mL beaker containing the ice. Stir
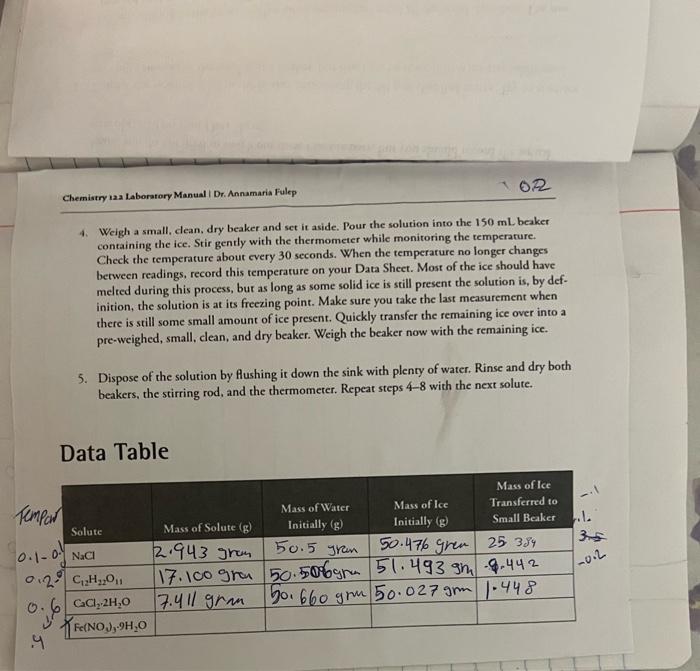
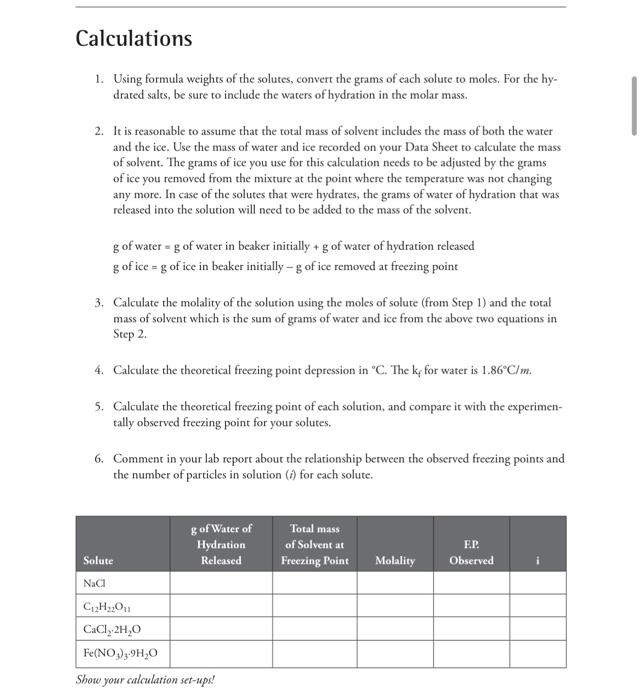
4. Weigh a small, dean, dry beaker and set it aside. Pour the solution into the 150mL beaker containing the ice. Stir gently with the thermometer while monitoring the temperature. Check the temperature about every 30 seconds. When the temperature no longer changes between readings, record this temperature on your Data Sheet. Most of the ice should have melted during this process, but as long as some solid ice is still present the solution is, by definition, the solution is at its freezing point. Make sure you take the last measurement when there is still some small amount of ice present. Quickly transfer the remaining ice over into a pre-weighed, small, clean, and dry beaker. Weigh the beaker now with the remaining ice. 5. Dispose of the solution by flushing it down the sink with plenty of water. Rinse and dry both beakers, the stirring rod, and the thermometer. Repeat steps 4-8 with the next solute. Data Table 1. Using formula weights of the solutes, convert the grams of each solute to moles. For the hydrated salts, be sure to include the waters of hydration in the molar mass. 2. It is reasonable to assume that the total mass of solvent includes the mass of both the water and the ice. Use the mass of water and ice recorded on your Data Sheet to calculate the mass of solvent. The grams of ice you use for this calculation needs to be adjusted by the grams of ice you removed from the mixture at the point where the temperature was not changing any more. In case of the solutes that were hydrates, the grams of water of hydration that was released into the solution will need to be added to the mass of the solvent. g of water =g of water in beaker initially +g of water of hydration released g of ice =g of ice in beaker initially g of ice removed at freezing point 3. Calculate the molality of the solution using the moles of solute (from Step 1) and the total mass of solvent which is the sum of grams of water and ice from the above two equations in Step 2. 4. Calculate the theoretical freezing point depression in C. The kf for water is 1.86C/m. 5. Calculate the theoretical freezing point of each solution, and compare it with the experimentally observed freezing point for your solutes. 6. Comment in your lab report about the relationship berween the observed freczing points and the number of particles in solution ( i ) for each solute. Show your calculation set-ups
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


