Question: I need help answering this one problem using the given values please with step by step help, for a thumbsup. --Given Values-- Concentration of Nitrogen

 I need help answering this one problem using the given values please with step by step help, for a thumbsup.
I need help answering this one problem using the given values please with step by step help, for a thumbsup.
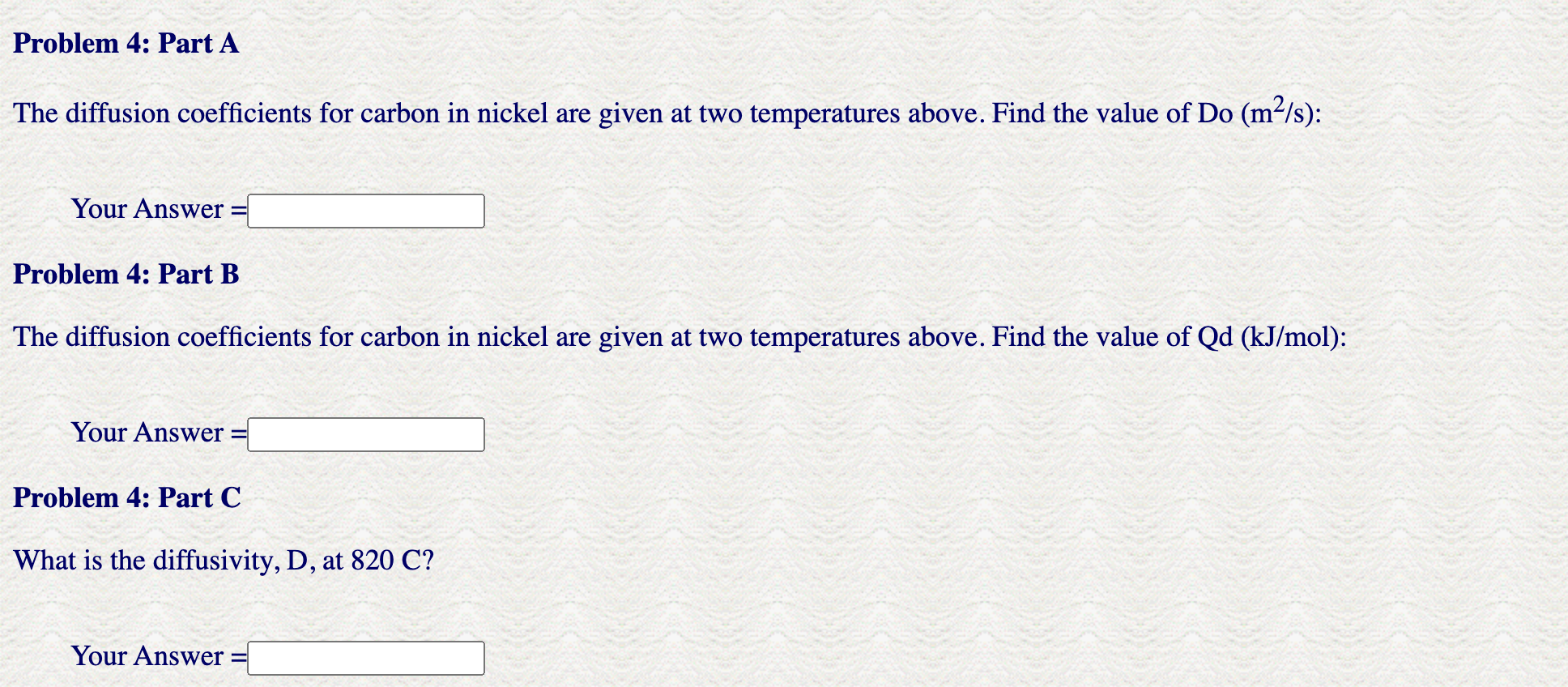
--Given Values-- Concentration of Nitrogen (wt\%): =0.24 Position in mm=4.2 Diffusion Coefficient for Nitrogen in Iron at 700C=6.30E10 Nitrogen in Iron Heat Treatment Time (hours): =19 Carburizing Time (hrs): =6 Steel Alloy Case Depth (mm):=3.7 Steel Alloy New Case Depth (mm)=7.9 Activation Energy kJ/mol:=102 Temperature for Problem 3 (Celsius): =814 - = Data for Problem 4 Temperature in C:=600 Diffusivity (m2/s)=5.24E14 Temperature in C:=705 Diffusivity (m2/s)=3.76E13 The diffusion coefficients for carbon in nickel are given at two temperatures above. Find the value of Do (m2/s) : Your Answer = Problem 4: Part B The diffusion coefficients for carbon in nickel are given at two temperatures above. Find the value of Qd(kJ/mol) : Your Answer = Problem 4: Part C What is the diffusivity, D, at 820C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


