Question: I need help! Can someone do ALL 21-25 please. Thank you! 21. Consider the following reaction: 4NH3(g)+7O2(g)2N2O4(g)+6H2O(g) If the initial concentrations of NH3(g) and O2(g)
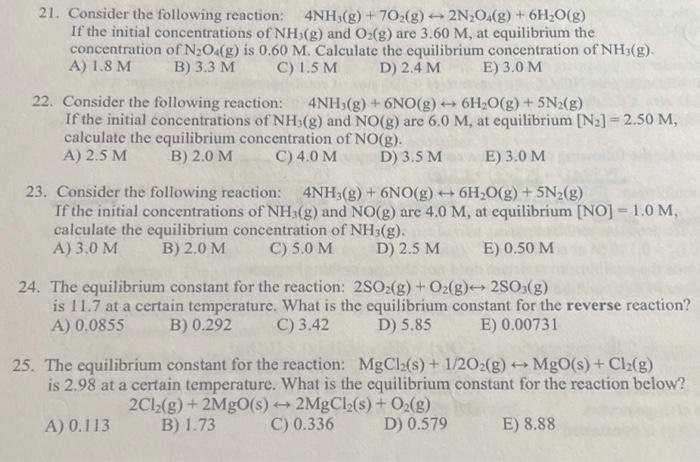
21. Consider the following reaction: 4NH3(g)+7O2(g)2N2O4(g)+6H2O(g) If the initial concentrations of NH3(g) and O2(g) are 3.60M, at equilibrium the concentration of N2O4(g) is 0.60M. Calculate the equilibrium concentration of NH3(g). A) 1.8M B) 3.3M C) 1.5M D) 2.4M E) 3.0M 22. Consider the following reaction: 4NH3(g)+6NO(g)6H2O(g)+5N2(g) If the initial concentrations of NH3(g) and NO(g) are 6.0M, at equilibrium [N2]=2.50M, calculate the equilibrium concentration of NO(g). A) 2.5M B) 2.0M C) 4.0M D) 3.5M E) 3.0M 23. Consider the following reaction: 4NH3(g)+6NO(g)6H2O(g)+5N2(g) If the initial concentrations of NH3(g) and NO(g) are 4.0M, at equilibrium [NO]=1.0M, calculate the equilibrium concentration of NH3(g). A) 3.0M B) 2.0M C) 5.0M D) 2.5M E) 0.50M 24. The equilibrium constant for the reaction: 2SO2(g)+O2(g)2SO3(g) is 11.7 at a certain temperature. What is the equilibrium constant for the reverse reaction? A) 0.0855 B) 0.292 C) 3.42 D) 5.85 E) 0.00731 25. The equilibrium constant for the reaction: MgCl2(s)+1/2O2(g)MgO(s)+Cl2(g) is 2.98 at a certain temperature. What is the equilibrium constant for the reaction below? A) 0.113 2Cl2(g)+2MgO(s)2MgCl2(s)+O2(g) B) 1.73 C) 0.336 D) 0.579 E) 8.88
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


